Comparative dynamics of NMDA- and AMPA-glutamate receptor N-terminal domains
By Anindita Dutta, Indira H. Shrivastava, Madhav Sukumaran, Ingo H. Greger and Ivet Bahar.
Published in Structure 20(11): 1838-1849, on September 6, 2012. PMID: 22959625. PMCID: PMC3496038. Link to publication page.
Core Facility: Computational Modeling
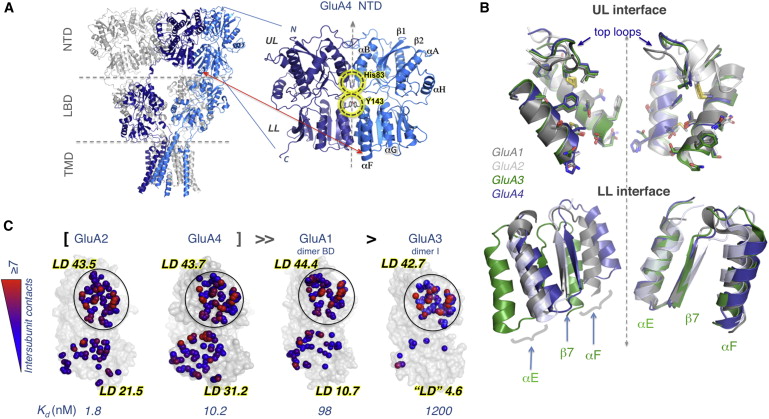
Figure 1: Structure of the GluA4 NTD Facilitates a Comparative Structural Analysis. (A) Intact structure of GluA2 AMPAR (left) displaying the spatial arrangements of four subunits (two shown in gray and the others in blue and dark blue) that span the three domains (NTD, LBD, and TMD). The location of the NTD dimer resolved for GluA4 is enlarged. Subunits are symmetrically positioned, each consisting of an upper lobe (UL) and a lower lobe (LL); secondary structural features (helices αA, αB, αE, αF, αG, and αH and strands β1 and β2) are labeled. Interfacial interactions are highlighted.(B) UL dimerization interfaces of GluA1–A4 are largely conserved but LL packing shows heterogeneity. UL interfaces of GluA1–GluA2 (grays), GluA3 (green), and GluA4 (blue) have been artificially separated to show the structural conservation and orientations of key residues (shown in stick) making contacts across the interface. Two-fold axis of symmetry is shown as a dashed line. Superposition of LL shows distinct differences in interface packing that is most prominent in GluA3.(C) Intersubunit contacts at the UL and LL interfaces of GluA1–A4 NTDs. Atoms making interfacial contacts within 4.5Å are shown as spheres and colored from blue (one contact) to red (≥7 contacts). Calculated local contact density (LD) indices and empirically measured dimer dissociation constants (Kd) are also shown. The four NTDs are ranked by their homodimerization affinity. Note the LL interface is highly variable between AMPAR paralogs, whereas the UL interface is largely invariant.See also Figure S1 and Tables 1 and S1.
Abstract
Ionotropic glutamate receptors (iGluRs) harbor two extracellular domains: the membrane-proximal ligand-binding domain (LBD) and the distal N-terminal domain (NTD). These are involved in signal sensing: the LBD binds L-glutamate, which activates the receptor channel. Ligand binding to the NTD modulates channel function in the NMDA receptor subfamily of iGluRs, which has not been observed for the AMPAR subfamily to date. Structural data suggest that AMPAR NTDs are packed into tight dimers and have lost their signaling potential. Here, we assess NTD dynamics from both subfamilies, using a variety of computational tools. We describe the conformational motions that underly NMDAR NTD allosteric signaling. Unexpectedly, AMPAR NTDs are capable of undergoing similar dynamics; although dimerization imposes restrictions, the two subfamilies sample similar, interconvertible conformational subspaces. Finally, we solve the crystal structure of AMPAR GluA4 NTD, and combined with molecular dynamics simulations, we characterize regions pivotal for an as-yet-unexplored dynamic spectrum of AMPAR NTDs.


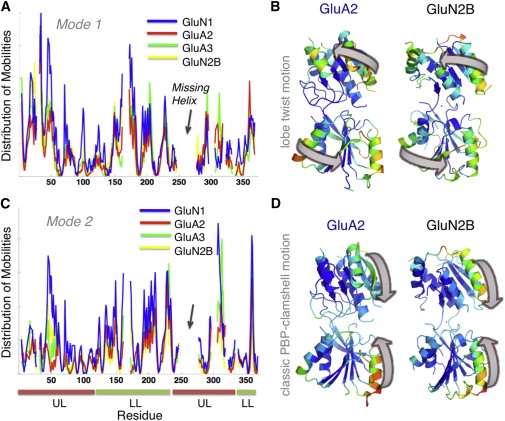
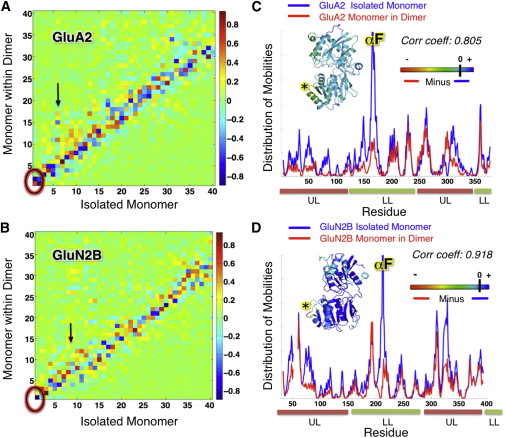
![Figure 2. Global Dynamics of GluA4 Dimer in Comparison to other AMPAR NTDs Probed by ANM(A) Distribution of square displacements of residues in the most global (lowest frequency) mode intrinsically accessible to AMPAR NTD dimers (GluA1-AC, GluA1-BD [3SAJ], GluA2-AB [3HSY], GluA3-CD [3O21], and GluA4 [PDB ID code 4GPA]). The four subtypes show similar profile (see the high correlations listed in Table S2) but different size motions (see Table S3).(B) Shared mechanism of global motion: counterrotation of the two protomers (indicated by red arrows), depicted for GluA4 as a representative structure, from the front and side views. The diagram is color-coded from red (most mobile in mode 1) to blue (least mobile). The global mobility rank of the four AMPAR NTD dimers is GluA3-CD (0.110) > GluA1-BD (0.169) > GluA1-AC (0.184) ≈ GluA4-BA (0.187) > GluA2-AB (0.187). The numbers in parentheses indicate the global mode eigenvalues (see Experimental Procedures).See also Table S1.](/site-media/images/publications/2012/bahar-sept2012-2-figure2.jpg)