Conformational dynamics of the nucleotide binding domains and the power stroke of a heterodimeric ABC transporter
By Smriti Mishra, Brandy Verhalen, Richard A. Stein, Po-Chao Wen, Emad Tajkhorshid, and Hassane Mchaourab.
Published in eLife 2014;10.7554/eLife.02740. May 16, 2014. PMCID: PMCID4046567.
Project: Structural Dynamics of ABC Transporter. Core Facility: Computational Modeling.
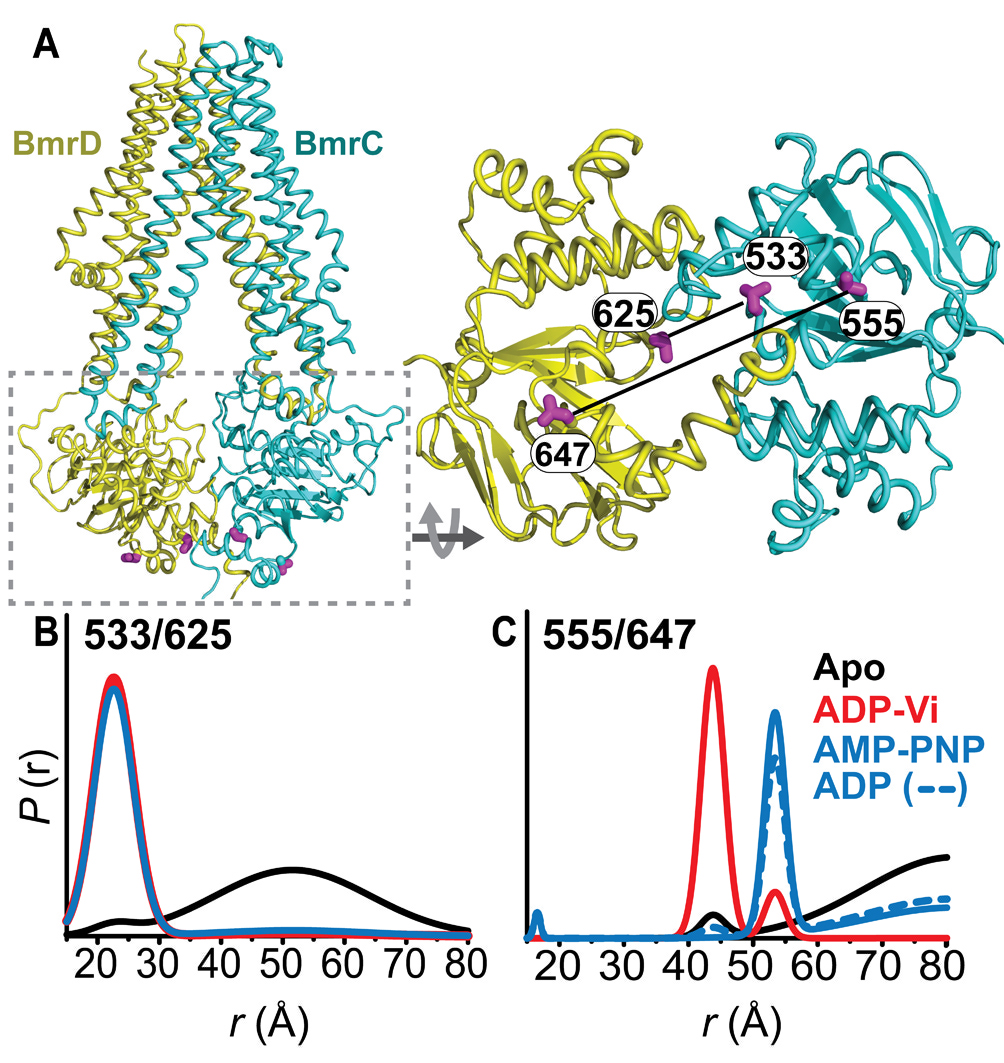
Figure 1. Conformational states of BmrCD NBDs. (A) Ribbon representation of BmrCD homology model showing the spin label pairs at symmetric sites across the NBD dimer interface (the transporter is rotated 90 degree and NBD interface is viewed from cytoplasm). (B) and (C) Distance distributions revealing conformational changes upon ADP and AMP-PNP binding (blue traces) and ATP hydrolysis (red traces). ATP binding mimicked by AMP-PNP induces the formation of an intermediate state distinct from that stabilized by ATP hydrolysis.
Abstract
Multidrug ATP binding cassette (ABC) exporters are ubiquitous ABC transporters that extrude cytotoxic molecules across cell membranes. Despite recent progress in structure determination of these transporters, the conformational motion that transduces the energy of ATP hydrolysis to the work of substrate translocation remains undefined. Here, we have investigated the conformational cycle of BmrCD, a representative of the heterodimer family of ABC exporters that have an intrinsically impaired nucleotide binding site. We measured distances between pairs of spin labels monitoring the movement of the nucleotide binding (NBD) and transmembrane domains (TMD). The results expose previously unobserved structural intermediates of the NBDs arising from asymmetric configuration of catalytically inequivalent nucleotide binding sites. The two-state transition of the TMD, from an inward- to an outward-facing conformation, is driven exclusively by ATP hydrolysis. These findings provide direct evidence of divergence in the mechanism of ABC exporters.