Conformational dynamics at the inner gate of KcsA during activation
By Raymond E. Hulse, Joseph R. Sachleben, Po-Chao Wen, Mahmoud Moradi, Emad Tajkhorshid, and Eduardo Perozo.
Published in Biochemistry on April 29, 2014;53(16):2557-9. PMID: 24621378. PMCID: PMC4010282. Link to publication page.
Project: Dynamics of Ion Permeation and Conformational Coupling in – KcsA
Abstract
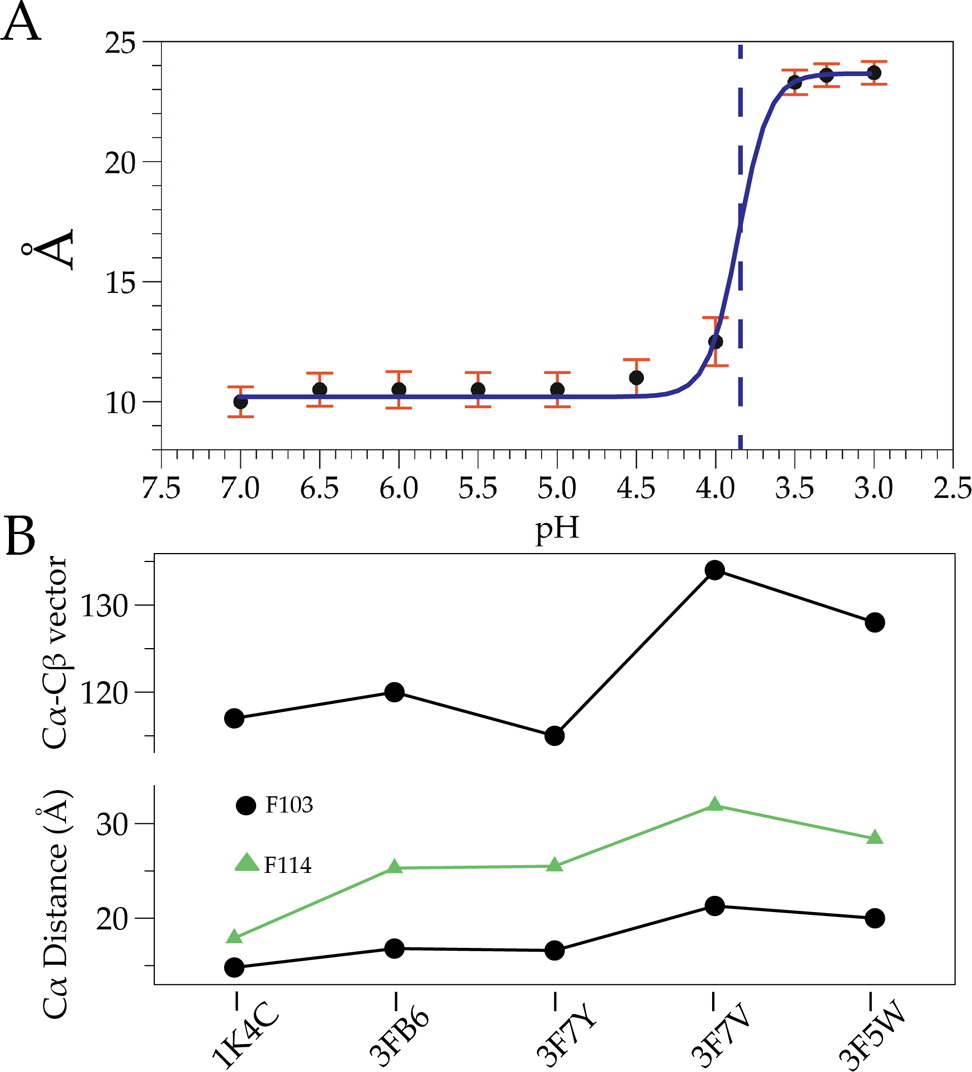
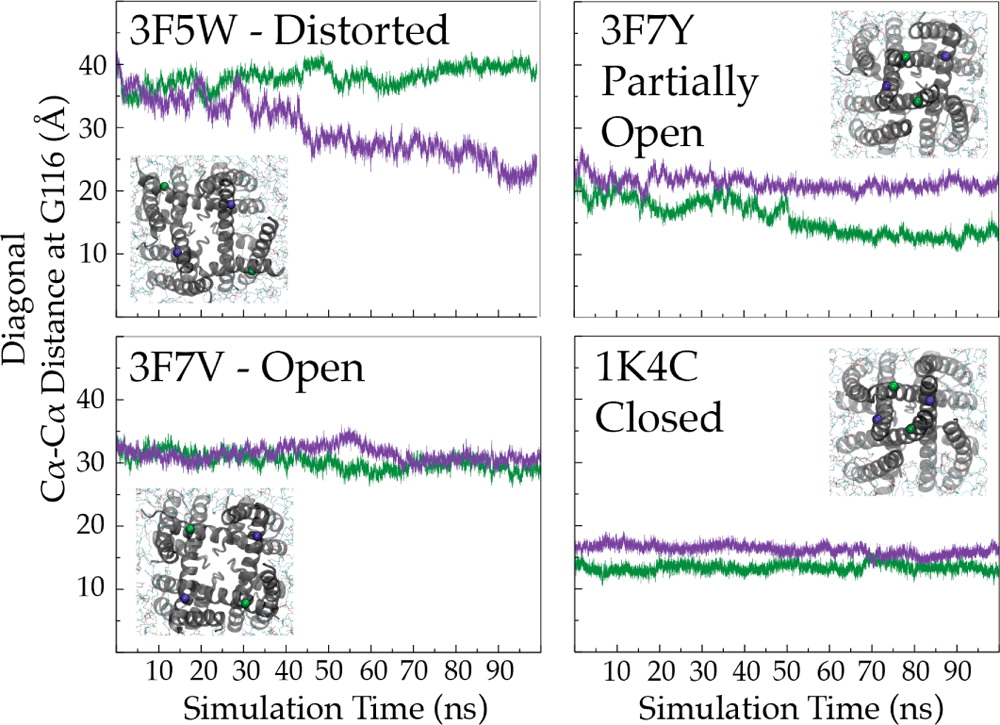
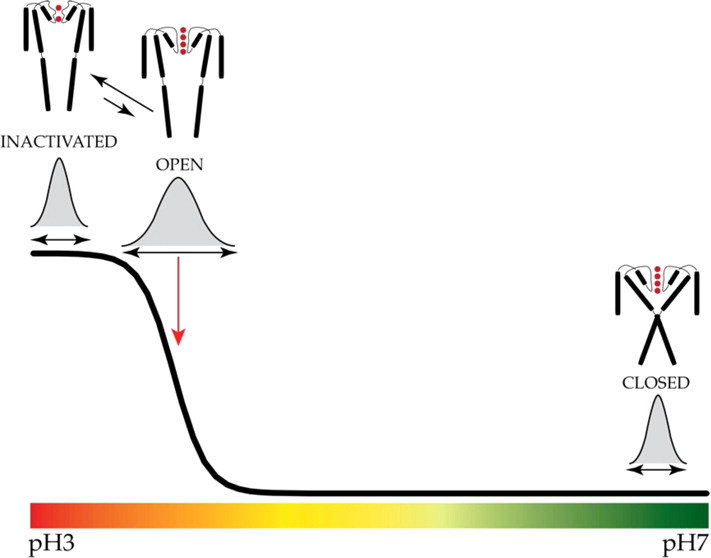
The potassium channel KcsA offers a unique opportunity to explicitly study the dynamics of the moving parts of ion channels, yet our understanding of the extent and dynamic behavior of the physiologically relevant structural changes at the inner gate in KcsA remains incomplete. Here, we use electron paramagnetic resonance, nuclear magnetic resonance, and molecular dynamics simulations to characterize the extent of pH-dependent conformational changes of the inner gate in lipid bilayers or detergent micelles. Our results show that under physiological conditions the inner gate experiences a maximal diagonal opening of 24 Å with the largest degree of dynamics near the pKa of activation (pH 3.9). These results extend the observation that the C-terminus is necessary to limit the extent of opening and imply that the inner gate regulates the extent of conformational change at the zone of allosteric coupling and at the selectivity filter.