PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein
By Peter J Hamilton, Andrea N Belovich, George Khelashvili, Christine Saunders, Kevin Erreger, Jonathan Javitch, Harald H Sitte, Harel Weinstein, Heinrich J G Matthies & Aurelio Galli.
Published in Nature Chemical Biology 2014 Jun 1 [Epub ahead of print] PMID: 24880859. Link to publication page.
MPSDC Project: The Transport Cycle in Neurotransmitter Uptake Systems
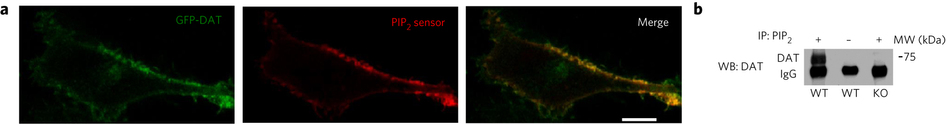
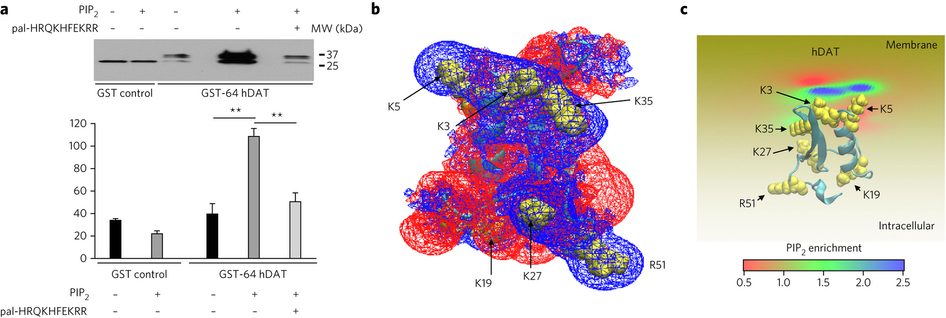
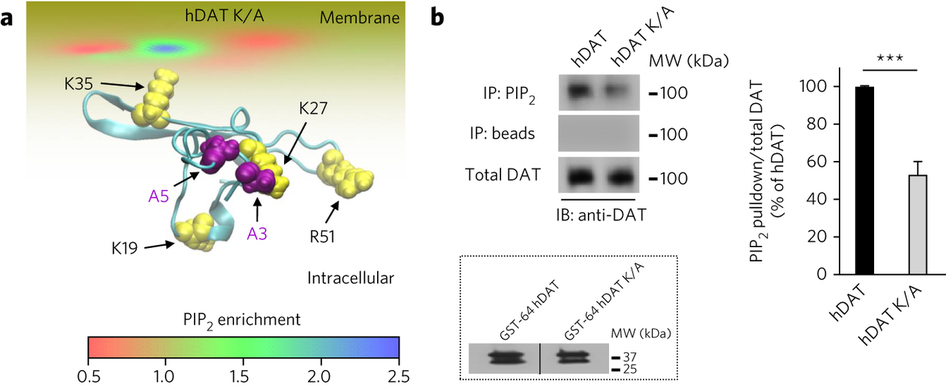
Figure 1. PIP2 interacts with hDAT. (a) hDAT and PIP2 colocalize at the plasma membrane. In hDAT-expressing cells, GFP-hDAT (green) colocalizes (yellow) at the plasma membrane with the PIP2 sensor PHPLCδ-mRFP (red; representative image from three live experiments with of 10−15 cells imaged per experiment). Scale bar, 5 μm. (b) PIP2 associates with endogenous mouse DAT in striatal tissue. In striatal lysate, DAT was detected in PIP2 immunoprecipitates (IP) using an anti-DAT antibody (+). DAT immunoreactivity was absent in the beads fraction (−) and in PIP2 immunoprecipitates from DAT KO animals (endogenous mouse IgG is observed; representative of n = 3). WB, western blot; WT, wild type; MW, molecular weight. The full blot is shown in Supplementary Figure 7.
Abstract
Phosphatidylinositol (4,5)-bisphosphate (PIP2) regulates the function of ion channels and transporters. Here, we demonstrate that PIP2 directly binds the human dopamine (DA) transporter (hDAT), a key regulator of DA homeostasis and a target of the psychostimulant amphetamine (AMPH). This binding occurs through electrostatic interactions with positively charged hDAT N-terminal residues and is shown to facilitate AMPH-induced, DAT-mediated DA efflux and the psychomotor properties of AMPH. Substitution of these residues with uncharged amino acids reduces hDAT-PIP2 interactions and AMPH-induced DA efflux without altering the hDAT physiological function of DA uptake. We evaluated the significance of this interaction in vivo using locomotion as a behavioral assay in Drosophila melanogaster. Expression of mutated hDAT with reduced PIP2 interaction in Drosophila DA neurons impairs AMPH-induced locomotion without altering basal locomotion. We present what is to our knowledge the first demonstration of how PIP2 interactions with a membrane protein can regulate the behaviors of complex organisms.