Structural Dynamics of ABC Transporter
Core Facilities: Membrane Protein Expression/Purification – Computational Modeling
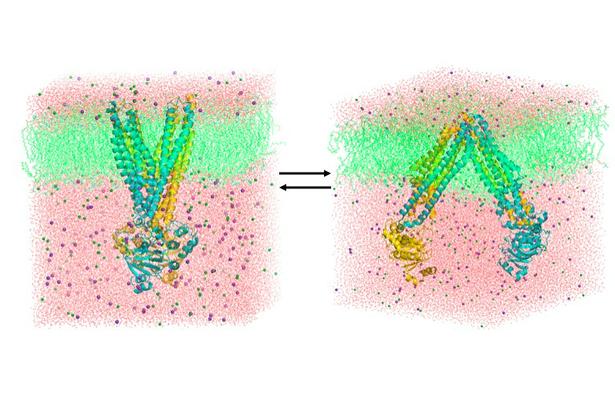
This Project will focus on the eukaryotic ABC transporter P-glycoprotein (Pgp, ABCB1), a human multidrug efflux system. The project aims to integrate computational, biochemical and spectroscopic approaches to understand Pgp transport mechanism, focusing on the relationship between ATP- and substrate-regulated reorientations in the transmembrane domain (TMD) and the cycles of dimerization and dissociation at the nucleotide binding domains (NBDs).
Project Description
This Project will focus on the eukaryotic ABC transporter P-glycoprotein (Pgp, ABCB1), a human multidrug efflux system. The project aims to integrate computational, biochemical and spectroscopic approaches to understand Pgp transport mechanism, focusing on the relationship between ATP- and substrate-regulated reorientations in the transmembrane domain (TMD) and the cycles of dimerization and dissociation at the nucleotide binding domains (NBDs).
This project seeks to develop an understanding of the structural dynamics of a class of multidrug transporters, the ATP binding cassette (ABC) transporters, that are associated with a number of human pathologies and play critical roles in the removal of cytotoxic agents. In multidrug resistance (MDR), the curative potential of chemotherapy is compromised by the development of resistance to a variety of cytotoxic anticancer agents. In “classical MDR” tumor cells, the ABC transporter, P-glycoprotein (Pgp or ABCB1, MDR1 gene product) acts as an ATP-dependent multidrug efflux pump conferring resistance to many cytotoxic anticancer drugs as well as to a variety of other hydrophobic compounds. MDR presents a key clinical obstacle in the treatment of cancer, epilepsy, malaria, and many other parasitic, bacterial and fungal infections. In humans, Pgp also plays direct roles in the absorption, distribution, and excretion of numerous pharmaceuticals, thus determining their effectiveness and toxicity. Furthermore, of the 48 human ABC transporters 17 have been implicated in human diseases. This project integrates computational, biochemical and spectroscopic approaches to understand the structural dynamics underlying the Pgp transport mechanism. Dr. Tajkhorshid will apply molecular dynamics (MD) simulations integrating experimental constraints to develop structural models for key conformational states and characterize their inter-conversion during the transport cycle. Drs. Al-Shawi and Nakamoto will use rapid mixing approaches to reveal the kinetic dimension of coupling of ATP hydrolysis to transport. Dr. Mchaourab will use spectroscopic approaches, primarily spin labeling, to determine the amplitude of conformational motion involved in the transduction process and provide experimental constraints for the molecular models.


