Conformational Transitions in P-class ATPases
Core Facilities: Membrane Protein Expression/Purification – Computational Modeling
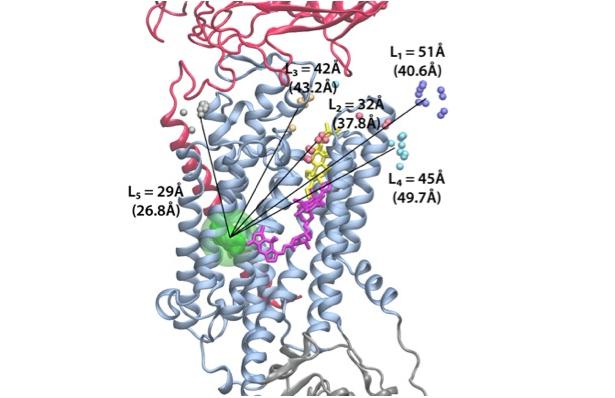
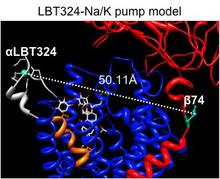
This project takes advantage of the available crystallographic snapshots of SERCA, and the recently solved first crystal structure of the Na/K pump as the baselines to explore ATP-driven ion translocation experimentally and computationally. This combined experimental and computational approach will reveal new insights on the conformational dynamics and energetic landscape associated with the pump’s catalytic and transport cycles.
Project Description
This project takes advantage of the available crystallographic snapshots of SERCA, and the recently solved first crystal structure of the Na/K pump as the baselines to explore ATP-driven ion translocation experimentally and computationally. This combined experimental and computational approach will reveal new insights on the conformational dynamics and energetic landscape associated with the pump’s catalytic and transport cycles.
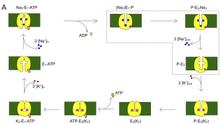
P-class (or E1-E2-type) ATPases constitute a superfamily of cation transport enzymes, present both in prokaryote and eukaryote, whose members mediate membrane flux of all common biologically relevant cations [1]. P-class pumps use ATP to transport ions against a gradient. The sarcoplasmic reticulum Ca2+-ATPase (SERCA) pumps 2 Ca2+ from the cytosol of muscle cells to the sarcoplasmic reticulum by exchanging H+. In each normal cycle, the Na/K pump transports 3 Na+ out of the cell by 2 K+ into the cell at the expense of the hydrolysis of one molecule of ATP. This extended project about P-class ATPases represents the combination of two projects, focused on the Na/K and SERCA pumps. From crystallographic snapshots of SERCA and the recently solved first crystal structure of the Na/K pump, we now dispose of a remarkable series of pictures showing how these enzymes look at different states of their transport cycle. SERCA is now by far the membrane protein where the most functionally different conformations have been described in precise structural detail. However, electrophysiological studies with SERCA are harder to do than with the Na/K pump, which can be expressed heterologously on the surface of cells. For this reason, while structural information about the Na/K pump is more limited (with only one x-ray structure available), the most informative functional analysis comes from experiments on the Na/K pump. In this proposal, we address questions about the Na/K and SERCA pumps: What are the conformational dynamics involved as the pump transits through conformational states revealed by x-ray crystallography? What is the nature of the coupling between the binding of ATP, phosphorylation, and the movements of charged species across the core of the protein? What are the stepwise voltage sensitive steps? What is the complete reaction pathway and what are the individual transition rates under various physiological conditions? Answers to these questions will correlate the structural changes to the function of membrane P-class ATPase pumps.