Dynamics of Ion Permeation and Conformational Coupling in – KcsA
Core Facilities: Membrane Protein Expression/Purification – Synthetic Antigen Binder (SAB) Generation and Crystallography – Computational Modeling
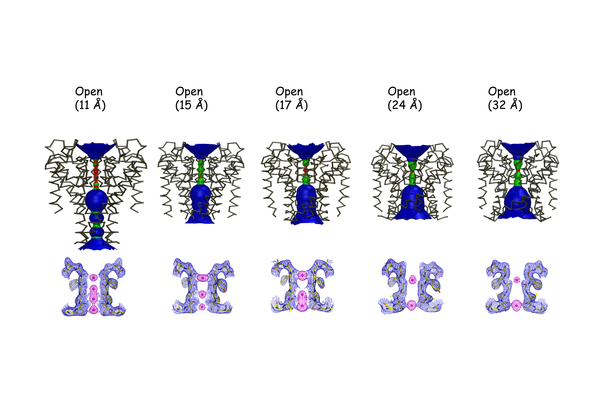
Correlation between inner gate opening and selectivity filter ion occupancy.Top, Structural classes of KcsA trapped in a a series of conformations corresponding to different dgrees of gate openings. In color is the calculated radius profile of the permeation pathway obtained with the program HOLE. Bottom, Electron density maps for the different K1 ion occupancies at the selectivity filter and various degrees of inner bundle gate opening (measured as Ca–Ca distances at Thr 112, top numbers). Shown, in each case, are the composite omit maps (blue mesh contoured at 2s) of the filter corresponding to residues 74–80 from two diagonally symmetrical subunits and the Fo2Fc omit map (magenta mesh contoured at 4–6 sigma) corresponding to ions in the filter
Project Description
Based on the semi-synthetic generation of KcsA, together with specific unnatural amino acid incorporation, this Project will focus, with unprecedented resolution, in the gating mechanisms taking place at the K+ channel selectivity filter. Starting from a number of recently determined structures representing snapshots of activation and inactivation intermediates, we will use a variety of spectroscopic and high-bandwith electrophysiology techniques to look into the process of ion permeation, activation and inactivation.
The molecular mechanism of gating in K+ channels encompasses two interrelated processes, the protein rearrangements that lead to channel opening and the energy transduction events that convert external stimuli (voltage, ligand binding, etc) into protein motion. We plan to study these events by combining spectroscopic, crystallographic, electrophysiological and computational methods to probe the conduction and inactivation gating in KcsA. Here, we intend to leapfrog the traditional structure-function studies by taking advantage of the aggregated expertise in this Consortium and the access to a superb set of Core facilities in a way that until now, no individual laboratory had been able to. The types of questions we plan to respond can be approached onlybecause of the robustness and experimental maturity of the system at hand (KcsA). Members of this Bridging Project have shown that KcsA represents an ideal system to study fundamental mechanisms of ion permeation and gating, and that these findings are directly applicable to eukaryotic systems. More recently, structure-function experiments led us to identify residues playing a critical role in C-type inactivation, flicker gating and pH sensitivity. Because of these findings, we are in a position to investigate the events linking the different conformations of the channel with the activation and inactivation processes at different time scales. We propose a concerted approach using semi-synthesis of KcsA, patch clamp, X-ray diffraction and a host of spectroscopic methods (EPR, NMR, and 2D-IR) to develop, with the aid of cutting edge computational methods, an understanding of the conformational changes taking place at the selectivity filter when it conducts ions and when it inactivates.