A synthetic antibody fragment targeting nicastrin affects assembly and trafficking of γ-secretase
By Xulun Zhang, Robert Hoey, Akiko Koide, Georgia Dolios, Marcin Paduch, Phuong Nguyen, Xianzhong Wu, Yueming Li, Steven L. Wagner, Rong Wang, Shohei Koide and Sangram S. Sisodia.
Published in J Biol Chem., 2014 Dec 12;289(50):34851-61.
PMID: 25352592. PMCID: PMC4263884. Link to publication page.
Core Facility: Synthetic Antigen Binder (SAB) Generation and Crystallography
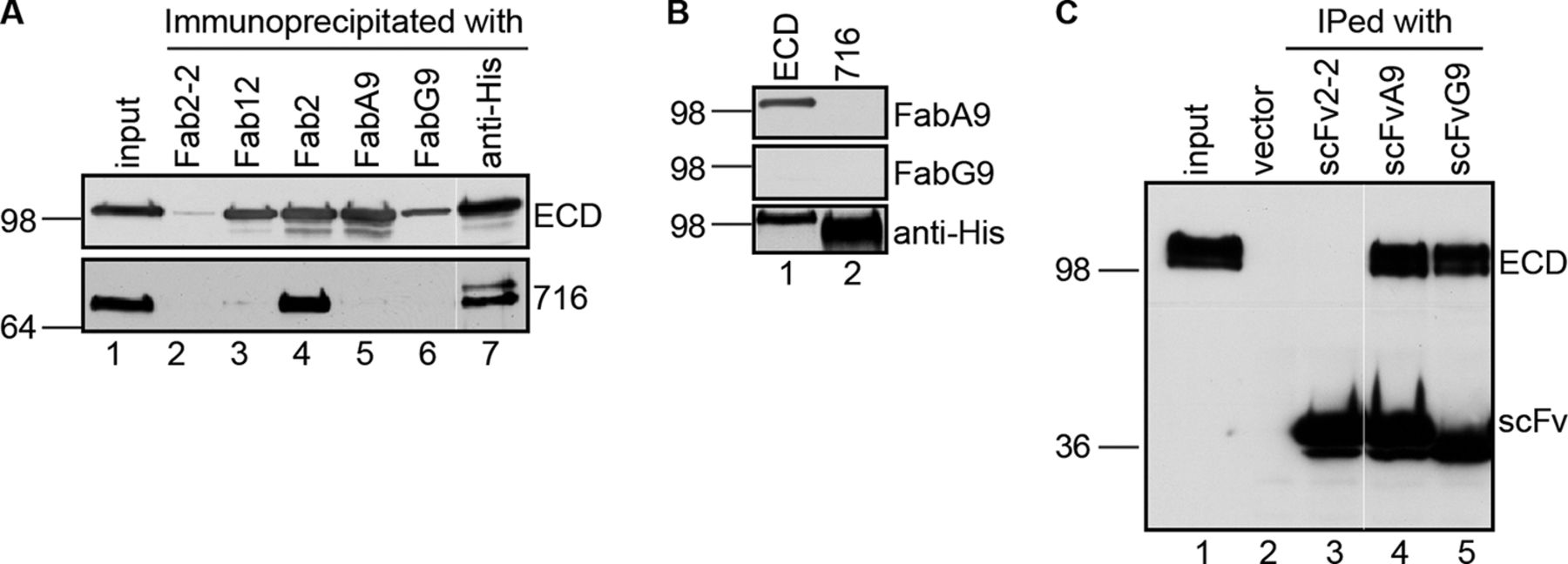
Figure 1. Generation and characterization of the NCT-specific single-chain variable fragment. A, immunoprecipitation of NCT full-length (ECD) or truncated (716) ectodomain with NCT-specific Fabs under native condition. Fabs 12, 2, A9, and G9 are NCT-specific Fabs. Fab2-2 is a negative control Fab. Anti-His antibody served as a positive control. Immunoprecipitated ECD and 716 were detected by anti-His6 antibody. A white line in each panel indicates the joining of lanes from different parts of the same gel. B, immunoblot analyses of purified ECD and 716 probed with FabA9, FabG9, or anti-His6 antibody. C, immunoprecipitation of ECD by scFvA9 and scFvG9. Immunoprecipitated (IPed) ECD and scFv were detected by anti-His6 antibody. A white line in each panel indicates the joining of lanes from different parts of the same gel.
Abstract
The γ-secretase complex, composed of presenilin, nicastrin (NCT), anterior pharynx-defective 1 (APH-1), and presenilin enhancer 2 (PEN-2), is assembled in a highly regulated manner and catalyzes the intramembranous proteolysis of many type I membrane proteins, including Notch and amyloid precursor protein. The Notch family of receptors plays important roles in cell fate specification during development and in adult tissues, and aberrant hyperactive Notch signaling causes some forms of cancer. γ-Secretase-mediated processing of Notch at the cell surface results in the generation of the Notch intracellular domain, which associates with several transcriptional coactivators involved in nuclear signaling events. On the other hand, γ-secretase-mediated processing of amyloid precursor protein leads to the production of amyloid β (Aβ) peptides that play an important role in the pathogenesis of Alzheimer disease. We used a phage display approach to identify synthetic antibodies that specifically target NCT and expressed them in the single-chain variable fragment (scFv) format in mammalian cells. We show that expression of a NCT-specific scFv clone, G9, in HEK293 cells decreased the production of the Notch intracellular domain but not the production of amyloid β peptides that occurs in endosomal and recycling compartments. Biochemical studies revealed that scFvG9 impairs the maturation of NCT by associating with immature forms of NCT and, consequently, prevents its association with the other components of the γ-secretase complex, leading to degradation of these molecules. The reduced cell surface levels of mature γ-secretase complexes, in turn, compromise the intramembranous processing of Notch.


