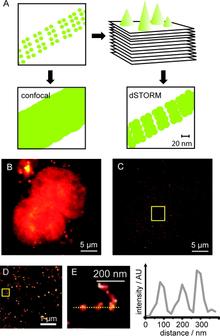
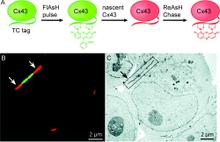
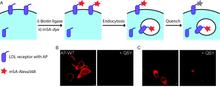
Chemical tags for labeling proteins inside living cells
By Chaoran Jing and Virginia Cornish
Published in Accounts of chemical research 44(9): 782-92 on September 20, 2011.
PMID: 21879706. PMCID: PMC3232020. Link to Pubmed page
Core Facility: Membrane Protein Expression/Purification
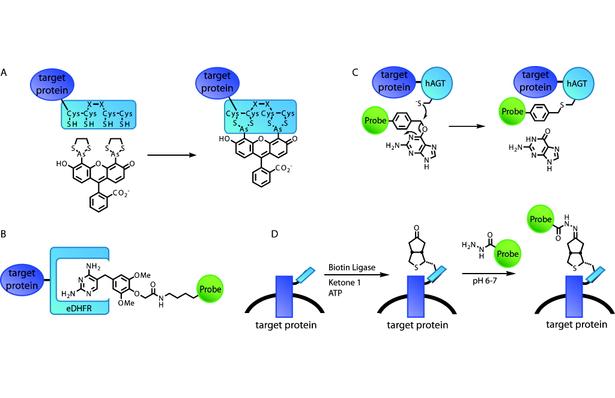
Figure 1. Schematic illustration of representative example technologies of different strategies for selectively labeling proteins in living cells. (A) The FlAsH tag features a short peptide with a tetracysteine core that directly binds bisarsenical fluorogens. (B) The eDHFR/TMP labeling strategy is based on noncovalent, high-affinity binding of TMP-probe heterodimers by the protein eDHFR. (C) In the SNAP-tag, the enzyme hAGT utilizes a guanine-probe heterodimer as a suicide substrate. (D) Biotin ligase enzymatically modifies a short peptide tag with a biotin analogue, which then reacts with the probe in a second step.
Abstract
To build on the last century’s tremendous strides in understanding the workings of individual proteins in the test tube, we now face the challenge of understanding how macromolecular machines, signaling pathways, and other biological networks operate in the complex environment of the living cell. The fluorescent proteins (FPs) revolutionized our ability to study protein function directly in the cell by enabling individual proteins to be selectively labeled through genetic encoding of a fluorescent tag. Although FPs continue to be invaluable tools for cell biology, they show limitations in the face of the increasingly sophisticated dynamic measurements of protein interactions now called for to unravel cellular mechanisms. Therefore, just as chemical methods for selectively labeling proteins in the test tube significantly impacted in vitro biophysics in the last century, chemical tagging technologies are now poised to provide a breakthrough to meet this century’s challenge of understanding protein function in the living cell. With chemical tags, the protein of interest is attached to a polypeptide rather than an FP. The polypeptide is subsequently modified with an organic fluorophore or another probe. The FlAsH peptide tag was first reported in 1998. Since then, more refined protein tags, exemplified by the TMP- and SNAP-tag, have improved selectivity and enabled imaging of intracellular proteins with high signal-to-noise ratios. Further improvement is still required to achieve direct incorporation of powerful fluorophores, but enzyme-mediated chemical tags show promise for overcoming the difficulty of selectively labeling a short peptide tag. In this Account, we focus on the development and application of chemical tags for studying protein function within living cells. Thus, in our overview of different chemical tagging strategies and technologies, we emphasize the challenge of rendering the labeling reaction sufficiently selective and the fluorophore probe sufficiently well behaved to image intracellular proteins with high signal-to-noise ratios. We highlight recent applications in which the chemical tags have enabled sophisticated biophysical measurements that would be difficult or even impossible with FPs. Finally, we conclude by looking forward to (i) the development of high-photon-output chemical tags compatible with living cells to enable high-resolution imaging, (ii) the realization of the potential of the chemical tags to significantly reduce tag size, and (iii) the exploitation of the modular chemical tag label to go beyond fluorescent imaging.