Chloride binding site of neurotransmitter sodium symporters
By Adriana K. Kantcheva, Matthias Quick, Lei Shi, Anne-Marie Lund Winther, Sebastian Stolzenberg, Harel Weinstein, Jonathan Javitch and Poul Nissen.
Published in Proceedings of the National Academy of Sciences of the United States of America q2013;110(21):8489-94. PMID: 23641004. PMCID: PMC3666746. Link to publication page.
Project: The Transport Cycle in Neurotransmitter Uptake Systems. Core Facility: Computational Modeling.
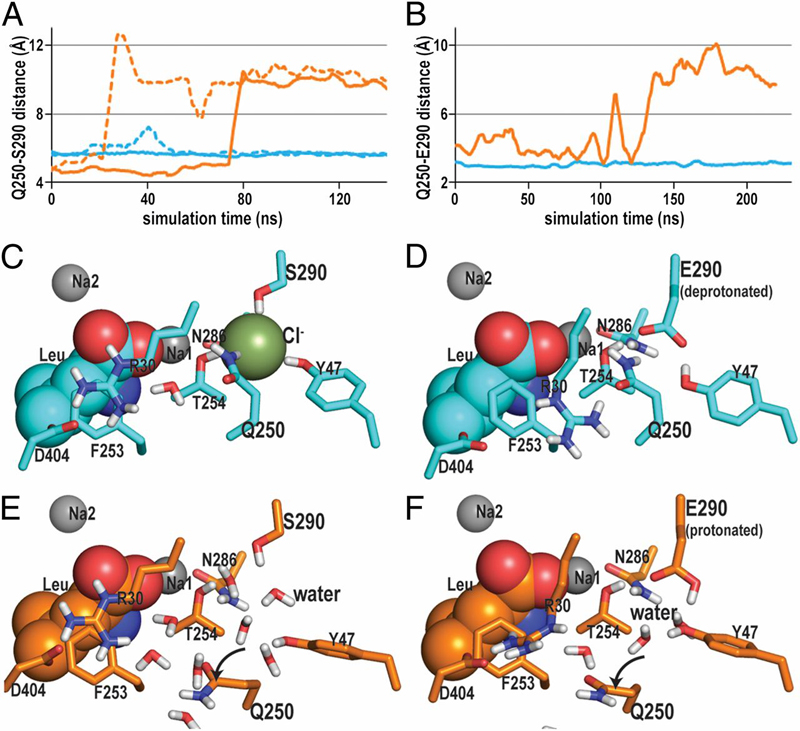
Figure 4. The impact of a negative charge on the interaction network near the Na1 and S1 binding sites revealed by MD simulations. A and B show the evolution of the distances between the closest heavy atoms of the residues at positions 250 and 290 in (A) the E290S mutant and (B) the WT in the course of the MD trajectories; the corresponding constructs are depicted in C–F. The curves in A (E290S mutant) are from simulations carried out in the presence or absence of Cl− (lines in cyan and orange, respectively), and the curves in B are for the corresponding constructs of the deprotonated (cyan) or protonated (orange) Glu290 in WT. Note that, in A, the dotted curves are from simulations with the n-octyl-β-glucopyranoside bound in the S2 site and found to reflect a similar trend as seen in the absence of n-octyl-β-glucopyranoside (solid curves). (C) In the E290S mutant, a negative charge provided by the bound Cl− ion is key in maintaining the interaction network near the Na1 and S1 binding sites. (D) In the WT, this function is fulfilled by the deprotonated Glu290. In the absence of such a negative charge, water molecules are seen to penetrate to this region in both the WT and the E290S mutant, interacting directly with the residue at position 290 as well as with Asn286 and Tyr47. This water entry results in an equivalent rotation of the Gln250 side chain in both (E) the E290S mutant and (F) the WT.
Abstract
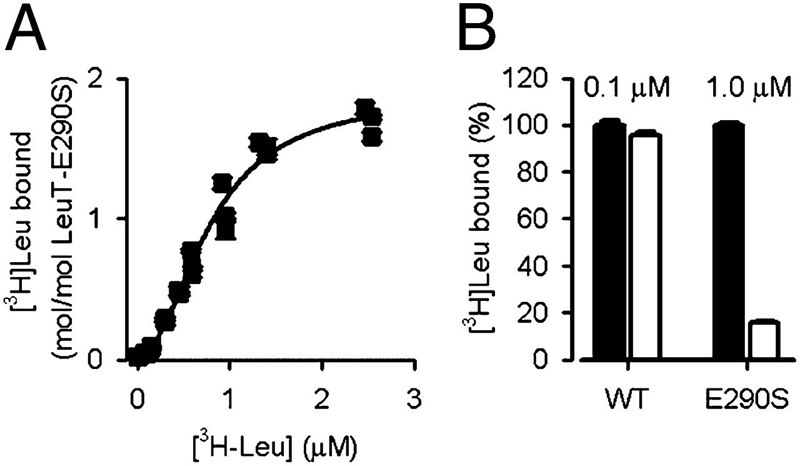
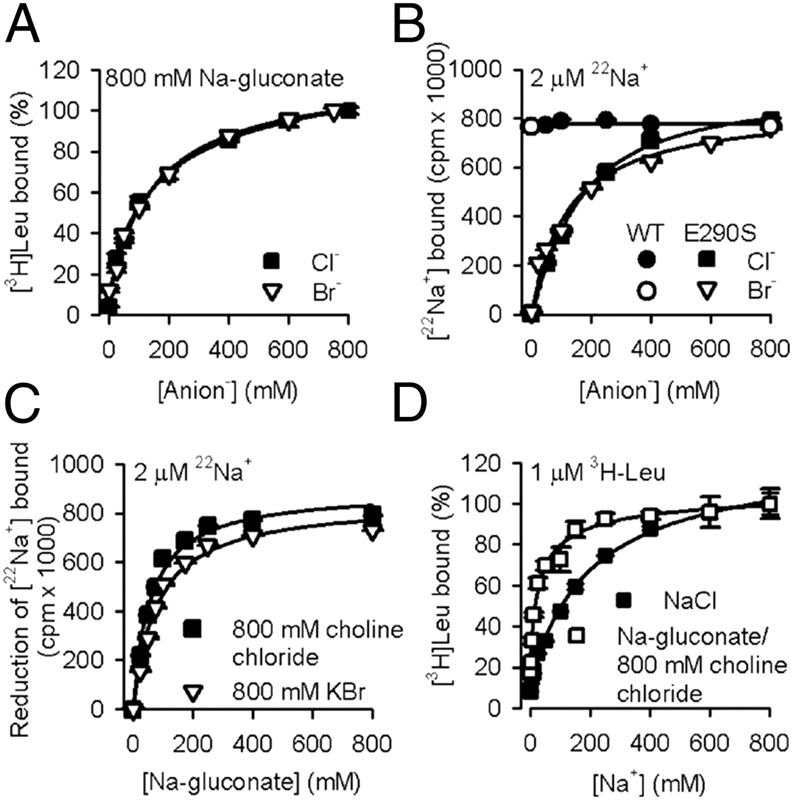
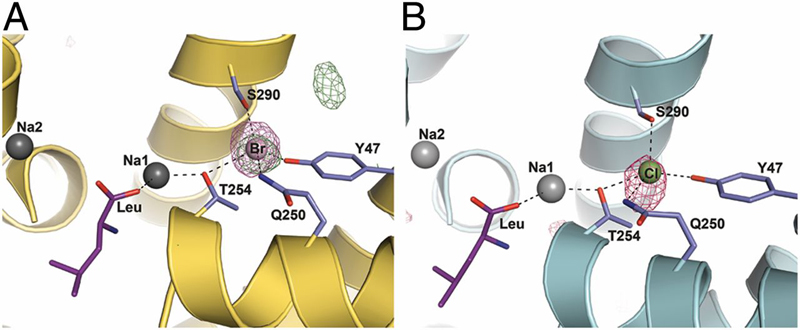
Neurotransmitter:sodium symporters (NSSs) play a critical role in signaling by reuptake of neurotransmitters. Eukaryotic NSSs are chloride-dependent, whereas prokaryotic NSS homologs like LeuT are chloride-independent but contain an acidic residue (Glu290 in LeuT) at a site where eukaryotic NSSs have a serine. The LeuT-E290S mutant displays chloride-dependent activity. We show that, in LeuT-E290S cocrystallized with bromide or chloride, the anion is coordinated by side chain hydroxyls from Tyr47, Ser290, and Thr254 and the side chain amide of Gln250. The bound anion and the nearby sodium ion in the Na1 site organize a connection between their coordinating residues and the extracellular gate of LeuT through a continuous H-bond network. The specific insights from the structures, combined with results from substrate binding studies and molecular dynamics simulations, reveal an anion-dependent occlusion mechanism for NSS and shed light on the functional role of chloride binding.