Large collective motions regulate the functional properties of glutamate transporter trimers
By Jie Jiang, Indira H. Shrivastava, Spencer D. Watts, Ivet Bahar, and Susan G. Amara
Published in the Proceedings of the National Academy of the Sciences of the United States of America 108(37): 15141-6 on September 13, 2011.
PMID: 21876140. PMCID: PMC3174670. Link to Pubmed page.
Core Facility: Computational Modeling
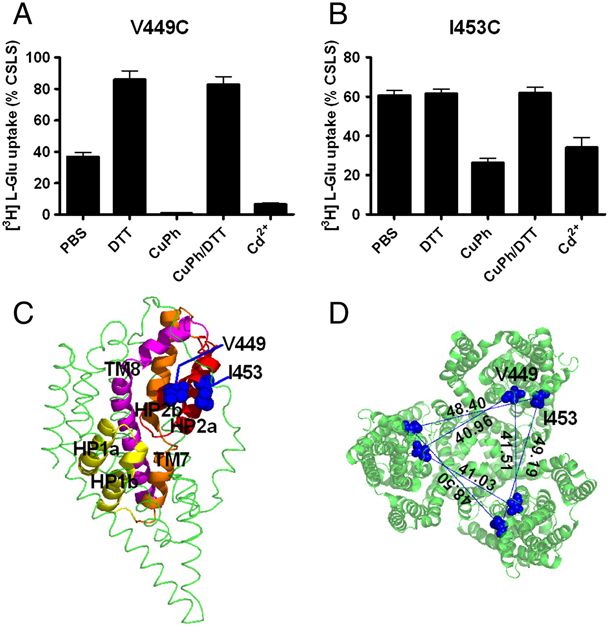
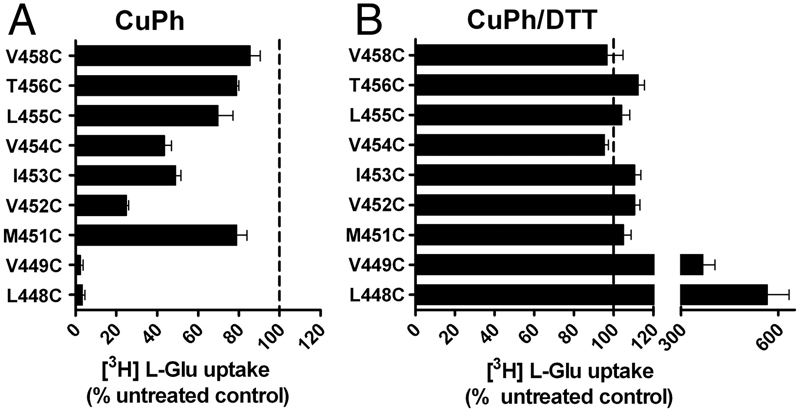
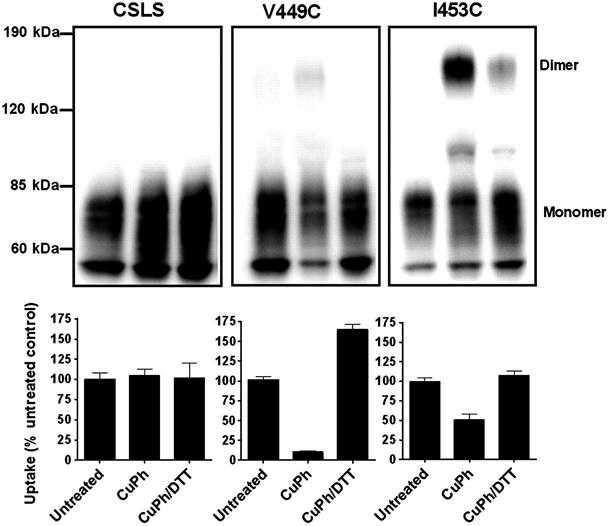
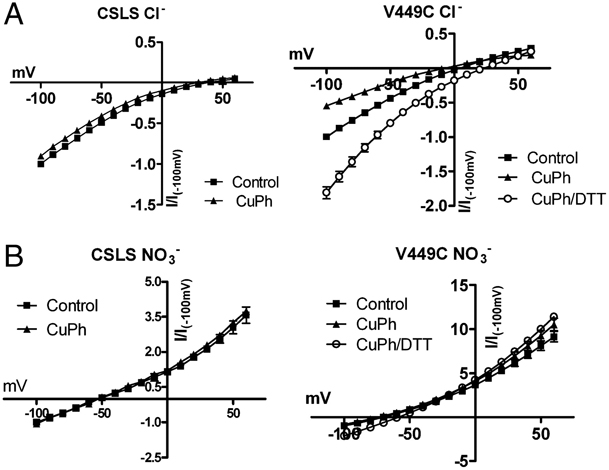
Figure 1. Inhibition of substrate transport by CuPh and cadmium in mutants V449C and I453C. COS7 cells were treated with 20 mM DTT, 300 μM CuPh, or CuPh followed by DTT, for 5 min. For CuPh preparation, CuSO4 and 1,10-phenanthroline were mixed in a 1∶2 ratio prior to use. Uptake assays were performed for 10 min at room temperature using 5 μM L-[3H] glutamate as substrate. The effect of cadmium on transport activity was determined by including 100 μM cadmium chloride in the uptake solution. Data for V449C (A) and I453C (B) are expressed as the percent of uptake activity relative to the CSLS control. (C) Locations of V449 and I453 on the helix H2b of the HP2 hairpin, shown for one subunit (side view). (D) Estimated distances between the three V449 residues, and the three I453 residues in an EAAT1 trimer (top view). The illustrations are based on the GltPh crystal structure in the outward-facing state (PDB ID 1XFH) and are made using the software Pymol.
Abstract
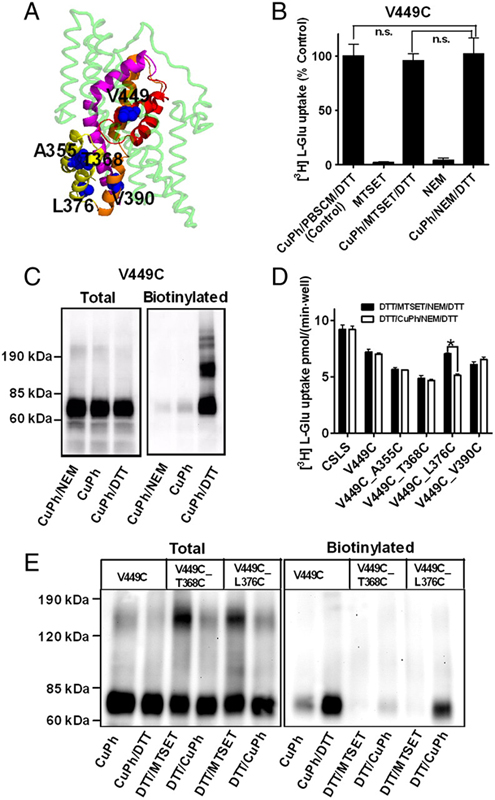
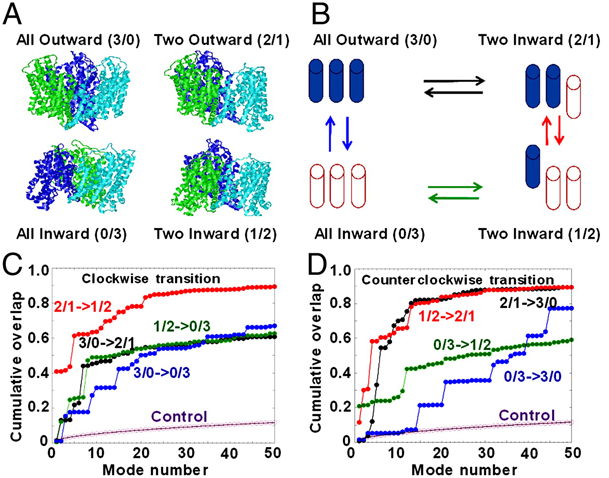
Glutamate transporters clear synaptically released glutamate to maintain precise communication between neurons and limit glutamate neurotoxicity. Although much progress has been made on the topology, structure, and function of these carriers, few studies have addressed large-scale structural motions collectively associated with substrate transport. Here we show that a series of single cysteine substitutions in the helical hairpin HP2 of excitatory amino acid transporter 1 form intersubunit disulfide cross-links within the trimer. After cross-linking, substrate uptake, but not substrate-activated anion conductance, is completely inhibited in these mutants. These disulfide bridges link residue pairs > 40 Å apart in the outward-facing crystal structure, and can be explained by concerted subunit movements predicted by the anisotropic network model (ANM). The existence of these global motions is further supported by the observation that single cysteine substitutions at the extracellular part of the transmembrane domain 8 can also be cross-linked by copper phenanthroline as predicted by the ANM. Interestingly, the transport domain in the un-cross-linked subunit of the trimer assumes an inward-facing orientation, suggesting that individual subunits potentially undergo separate transitions between outward- and inward-facing forms, rather than an all-or-none transition of the three subunits, a mechanism also supported by ANM-predicted intrinsic dynamics. These results shed light on how large collective motions contribute to the functional dynamics of glutamate transporters.