Molecular insights into the membrane-associated phosphatidylinositol 4-kinase IIα
By Qiangjun Zhou, Jiangmei Li, Hang Yu, Yujia Zhai, Zhen Gao, Yanxin Liu, Xiaoyun Pang, Lunfeng Zhang, Klaus Schulten, Fei Sun and Chang Chen.
Published in Nature Communications 5(3552) on March 28, 2014. PMID: 24675427. Link to publication page.
Core Facility: Computational Modeling
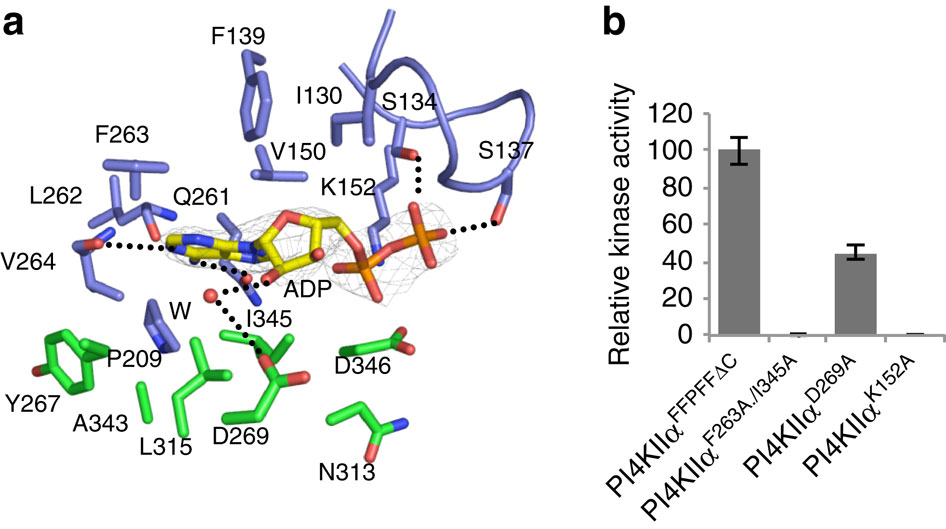
Figure 2. Nucleotide-binding pocket of PI4KIIα. (a) Interactions between ADP and PI4KIIα. Residues involved in ADP binding are labelled and shown as stick models with those from the N-lobe coloured in blue and those from the C-lobe coloured in green. A water molecule (W, red) is shown in sphere. The 2Fo-Fc electron density map is shown as a grey mesh and contoured at 1.8σ. Hydrogen bonds are shown as dashed lines. See also Table 2. (b) Kinase activities of PI4KIIα variants with mutations at the nucleotide-binding site. Kinase activity was measured in PI/Triton X-100 (0.2%) and normalized by using the activity of PI4KIIαFFPFFDC as 100%. The error bars represent the s.d. from three independent experiments.
Abstract
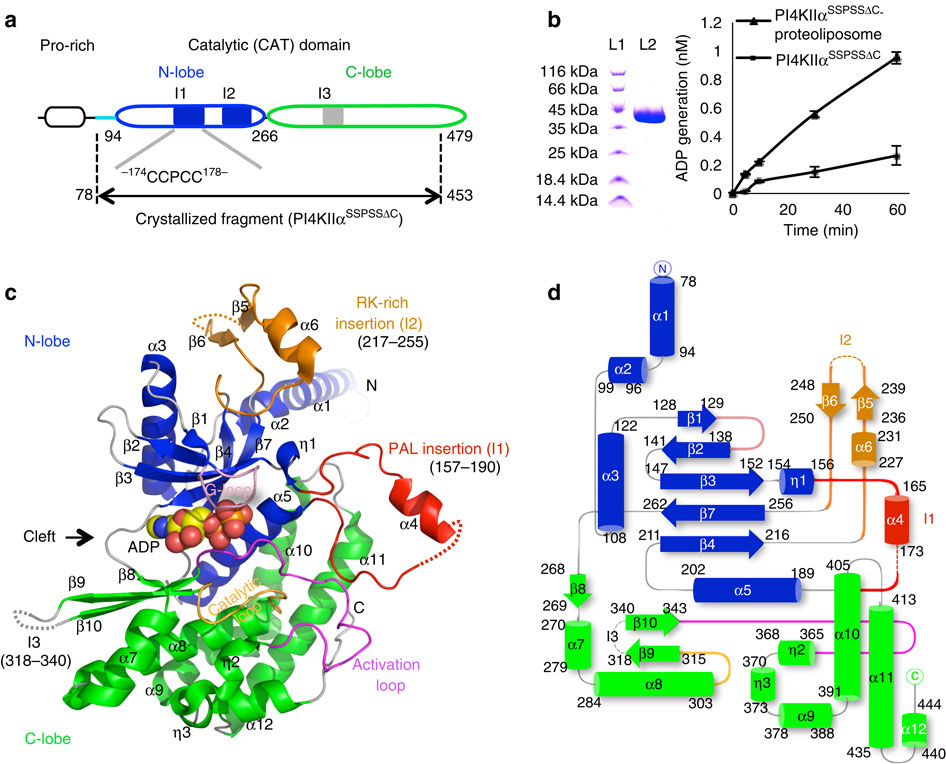
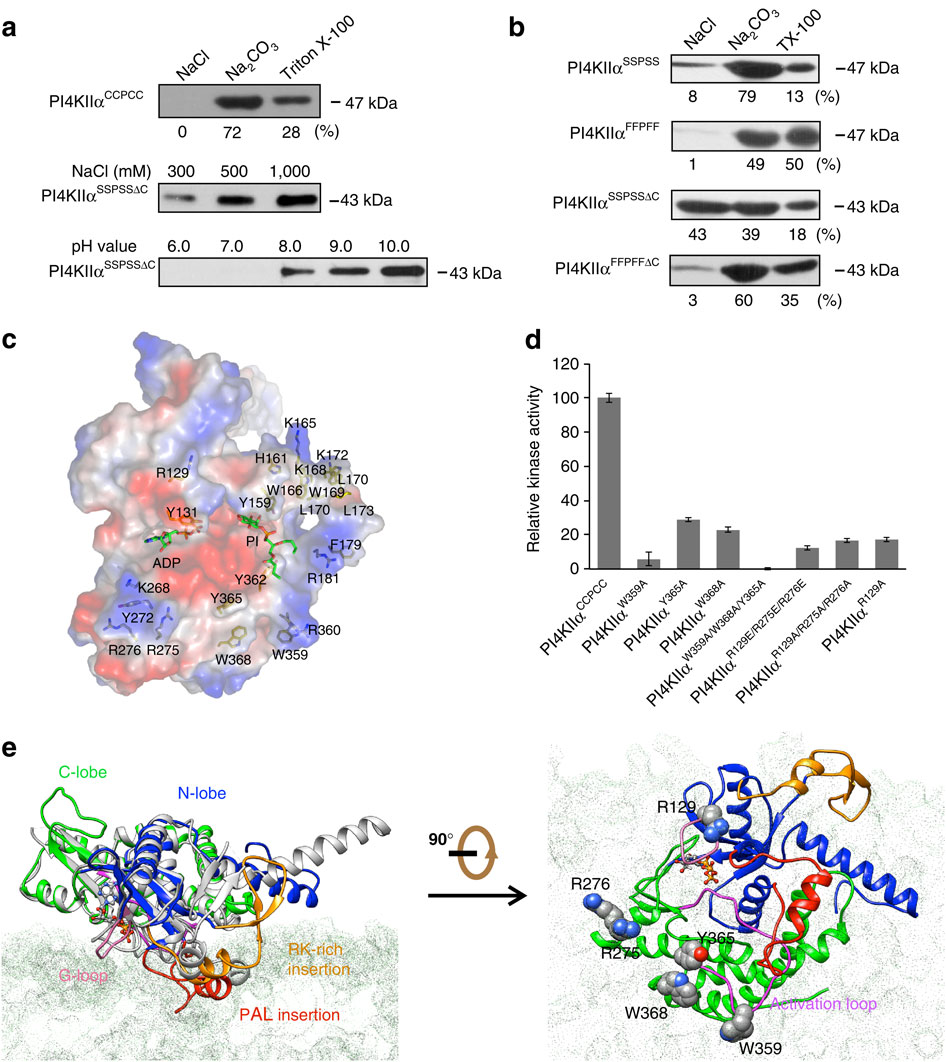
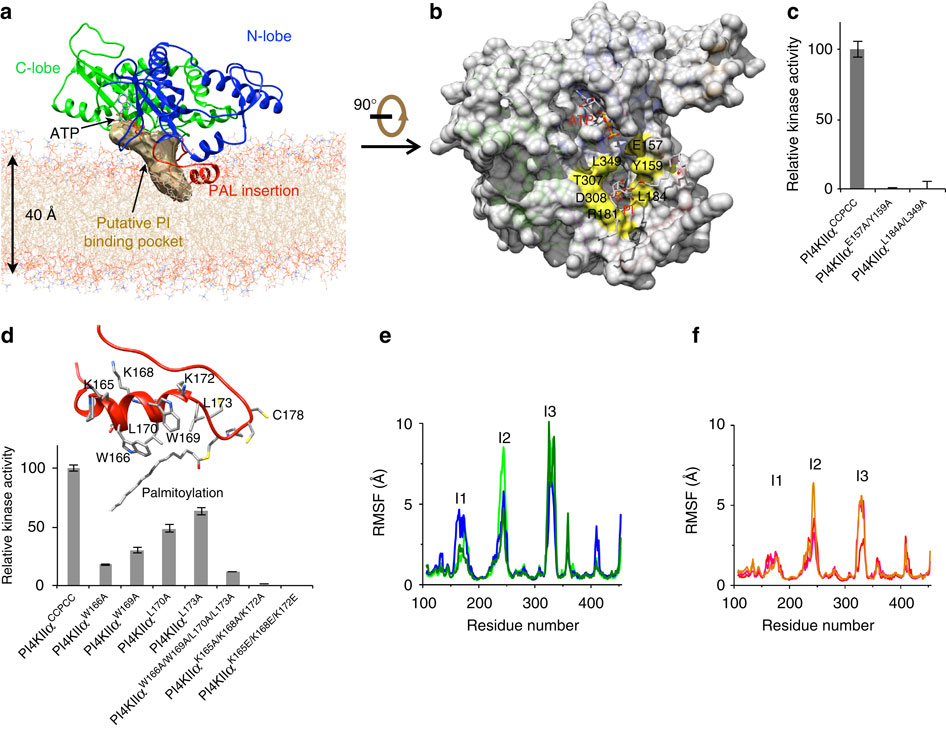
Phosphatidylinositol 4-kinase IIα (PI4KIIα), a membrane-associated PI kinase, plays a central role in cell signalling and trafficking. Its kinase activity critically depends on palmitoylation of its cysteine-rich motif (-CCPCC-) and is modulated by the membrane environment. Lack of atomic structure impairs our understanding of the mechanism regulating kinase activity. Here we present the crystal structure of human PI4KIIα in ADP-bound form. The structure identifies the nucleotide-binding pocket that differs notably from that found in PI3Ks. Two structural insertions, a palmitoylation insertion and an RK-rich insertion, endow PI4KIIα with the ‘integral’ membrane-binding feature. Molecular dynamics simulations, biochemical and mutagenesis studies reveal that the palmitoylation insertion, containing an amphipathic helix, contributes to the PI-binding pocket and anchors PI4KIIα to the membrane, suggesting that fluctuation of the palmitoylation insertion affects PI4KIIα’s activity. We conclude from our results that PI4KIIα’s activity is regulated indirectly through changes in the membrane environment.