NbIT – A New Information Theory-Based Analysis of Allosteric Mechanisms Reveals Residues that Underlie Function in the Leucine Transporter LeuT
By Michael V. LeVine and Harel Weinstein.
Published in PLoS Computational Biology on May 1, 2014;10(5):e1003603. PMID: 24785005. PMCID: PMC4006702. Link to publication page.
Project: The Transport Cycle in Neurotransmitter Uptake Systems. Core Facility: Computational Modeling.
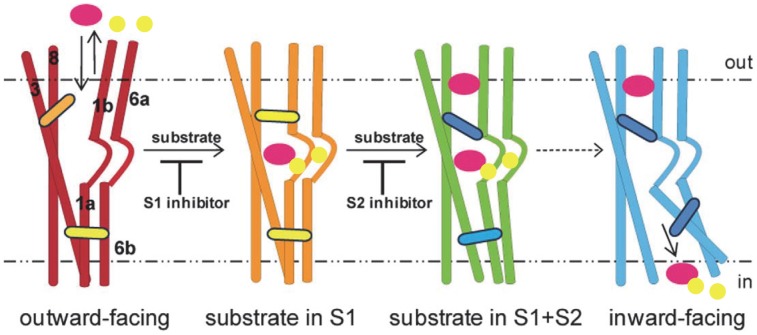
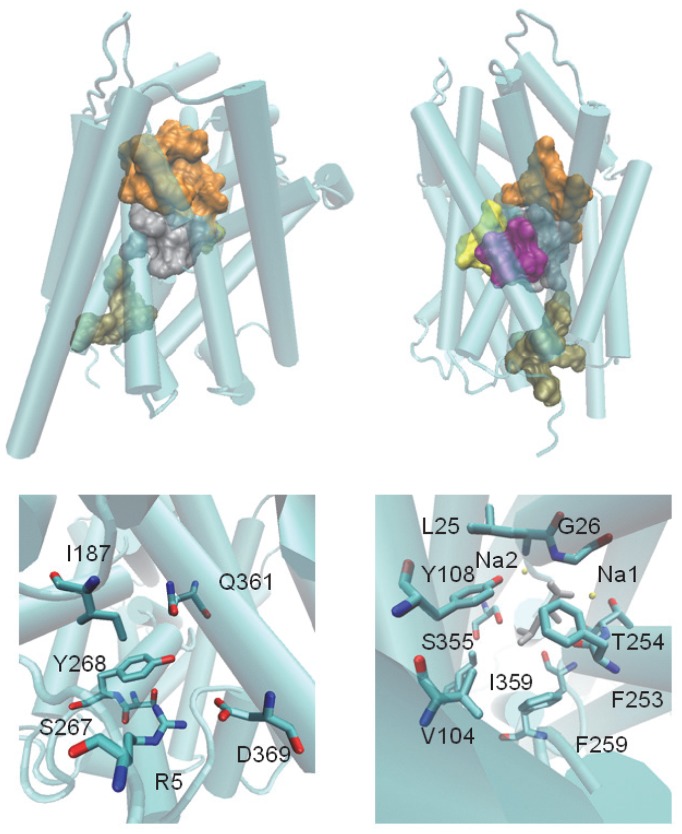
Figure 1. Representation of the states in the transport cycle of an NSS transporter. In this model, the transporter begins in an outward-open state (red), which can bind Na+ (yellow) and substrate (purple) in the primary site (S1) and then transition to a substrate-bound occluded state (orange). This state can bind in the S2 site, either inhibitors, such as TCAs, which block substrate release, or substrates (green), which produce release of Na+ and substrate from S1 (blue). Reproduced and modified with permission from [12].
Abstract
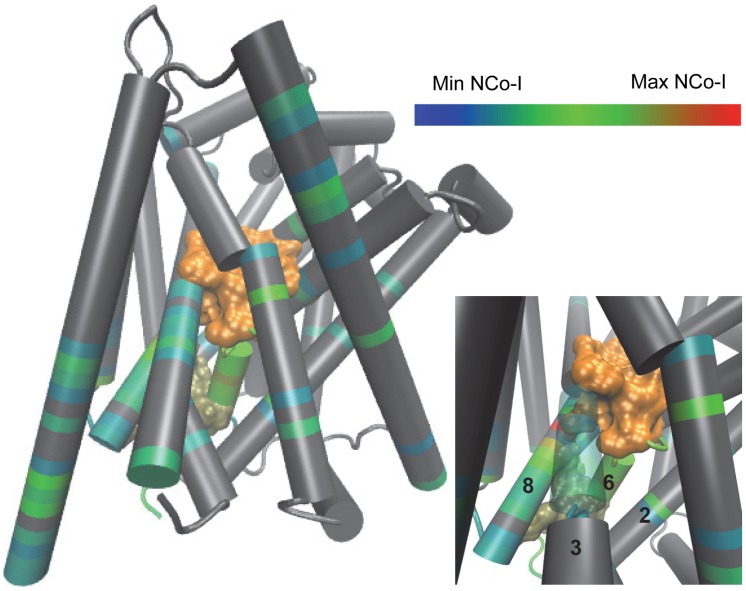
Complex networks of interacting residues and microdomains in the structures of biomolecular systems underlie the reliable propagation of information from an input signal, such as the concentration of a ligand, to sites that generate the appropriate output signal, such as enzymatic activity. This information transduction often carries the signal across relatively large distances at the molecular scale in a form of allostery that is essential for the physiological functions performed by biomolecules. While allosteric behaviors have been documented from experiments and computation, the mechanism of this form of allostery proved difficult to identify at the molecular level. Here, we introduce a novel analysis framework, called N-body Information Theory (NbIT) analysis, which is based on information theory and uses measures of configurational entropy in a biomolecular system to identify microdomains and individual residues that act as (i)-channels for long-distance information sharing between functional sites, and (ii)-coordinators that organize dynamics within functional sites. Application of the new method to molecular dynamics (MD) trajectories of the occluded state of the bacterial leucine transporter LeuT identifies a channel of allosteric coupling between the functionally important intracellular gate and the substrate binding sites known to modulate it. NbIT analysis is shown also to differentiate residues involved primarily in stabilizing the functional sites, from those that contribute to allosteric couplings between sites. NbIT analysis of MD data thus reveals rigorous mechanistic elements of allostery underlying the dynamics of biomolecular systems.