Structural basis for iron piracy by pathogenic Neisseria
By Nicholas Noinaj, Nicole C. Easley, Muse Oke, Naoko Mizuno, James Gumbart, Evzen Boura, Ashley N. Steere, Olga Zak, Philip Aisen, Emad Tajkhorshid, Robert W. Evans, Andrew R. Gorringe, Anne B. Mason, Alasdair C. Steven, and Susan K. Buchanan.
Published in Nature 483(7387): 53-58 on March 1, 2012.
PMID: 22327295. Link to Pubmed page.
Core Facility: Computational Modeling
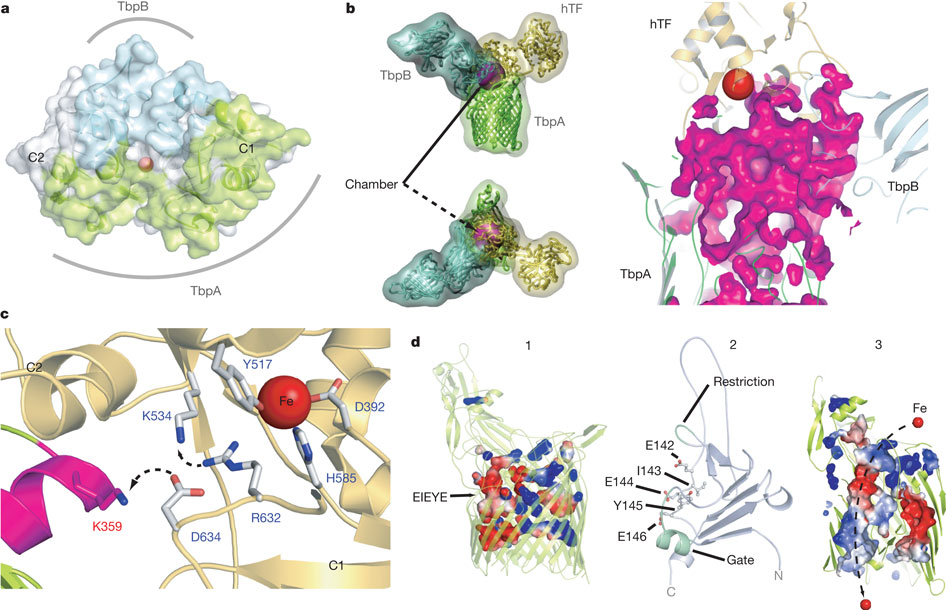
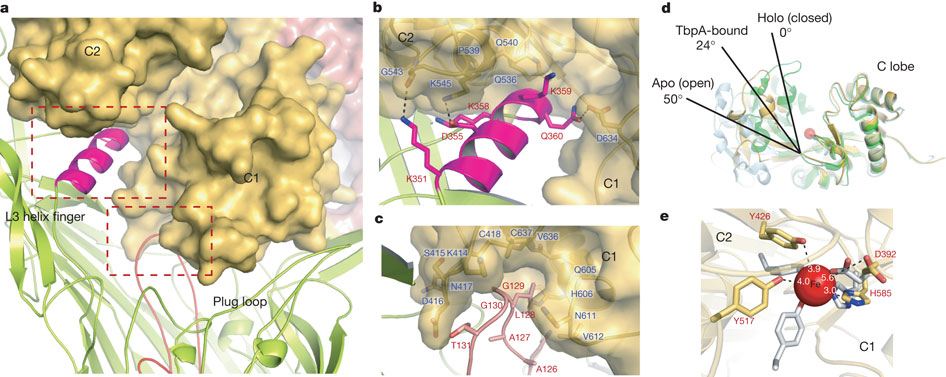
Figure 5: Mechanism for iron import. Binding surfaces of TbpA (green) and TbpB (cyan) mapped onto the hTF C lobe. b, Enclosed chamber formed by TbpA–TbpB–(holo)hTF (left, magenta sphere). A cutaway view (right) from inside the chamber illustrates the proximity of the iron (red). c, Model for iron release. Conserved K359 in the L3 helix finger is positioned to interact with residues that regulate iron release in eukaryotic iron uptake. d, Import of iron through TbpA. 1, an electrostatic surface depicts cavities between the TbpA barrel and plug domain; 2, plug domain constrictions close the tunnel; 3, molecular dynamics simulations show removal of constrictions upon interaction with TonB.
Abstract
Neisseria are obligate human pathogens causing bacterial meningitis, septicaemia and gonorrhoea. Neisseria require iron for survival and can extract it directly from human transferrin for transport across the outer membrane. The transport system consists of TbpA, an integral outer membrane protein, and TbpB, a co-receptor attached to the cell surface; both proteins are potentially important vaccine and therapeutic targets. Two key questions driving Neisseria research are how human transferrin is specifically targeted, and how the bacteria liberate iron from transferrin at neutral pH. To address these questions, we solved crystal structures of the TbpA–transferrin complex and of the corresponding co-receptor TbpB. We characterized the TbpB–transferrin complex by small-angle X-ray scattering and the TbpA–TbpB–transferrin complex by electron microscopy. Our studies provide a rational basis for the specificity of TbpA for human transferrin, show how TbpA promotes iron release from transferrin, and elucidate how TbpB facilitates this process.