Structural basis of lipid-driven conformational transitions in the KvAP voltage-sensing domain
By Qufei Li, Sherry Wanderling, Pornthep Sompornpisut & Eduardo Perozo.
Published in Nature Structural & Molecular Biology on January 12, 2014. E-Publication ahead of print. PMID: 24413055. Link to publication page.
Core Facility: Membrane Protein Expression/Purification
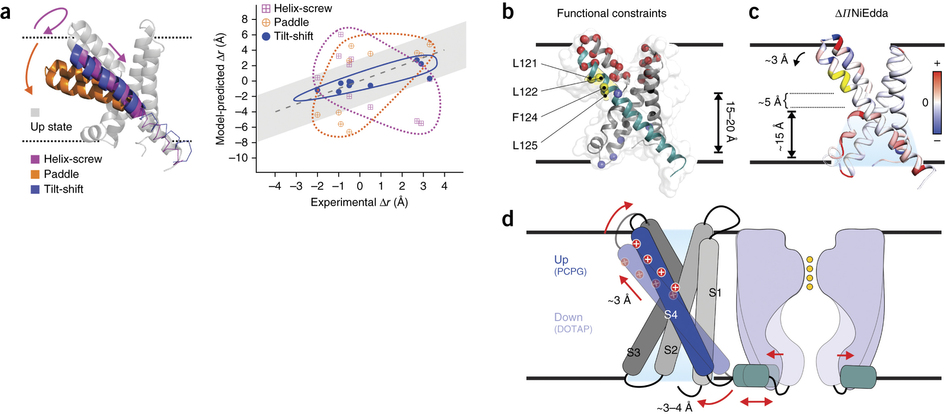
Figure 5. The tilt-shift model. (a) Evaluation of the distance changes, from up state to down state, derived from different gating models in the KvAP VSD. Left, ribbon representation of the up-state VSD model (gray) after MD equilibration and overlapped with putative models based on the helix-screw (magenta), paddle (orange) and tilt-shift (blue) gating mechanisms. Right, expected distance changes against experimental distance differences for the three down states. Gray region indicates the uncertainty of measurement. (b) An alternative explanation for the existing KvAP biotin-streptavidin trapping experiments. All the established state-dependent accessibilities on KvAP focused on the region of 121–125 (in yellow). The paddle model predicts a 15-Å to 20-Å downward S4 movement to account for the streptavidin trapping data (accessibility from the bottom is indicated). (c) ΔΠNiEdda mapped on to the crystal structure, showing water penetration deep into the bottom crevice, where the region 121–125 is only ∼5 Å above the uppermost NiEdda penetration. (d) Cartoon representation of the tilt-shift model on KvAP VSD. The ∼25° tilt and ∼2-Å downward shift of S4 could generate a movement of ∼3–4 Å of the S4-S5 linker, sufficient to open the pore.
Abstract
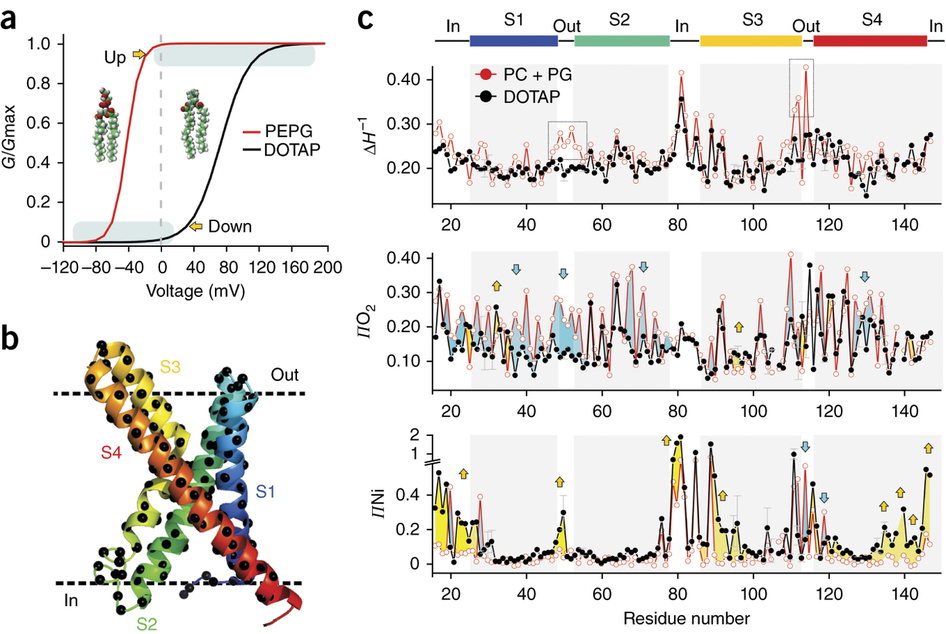
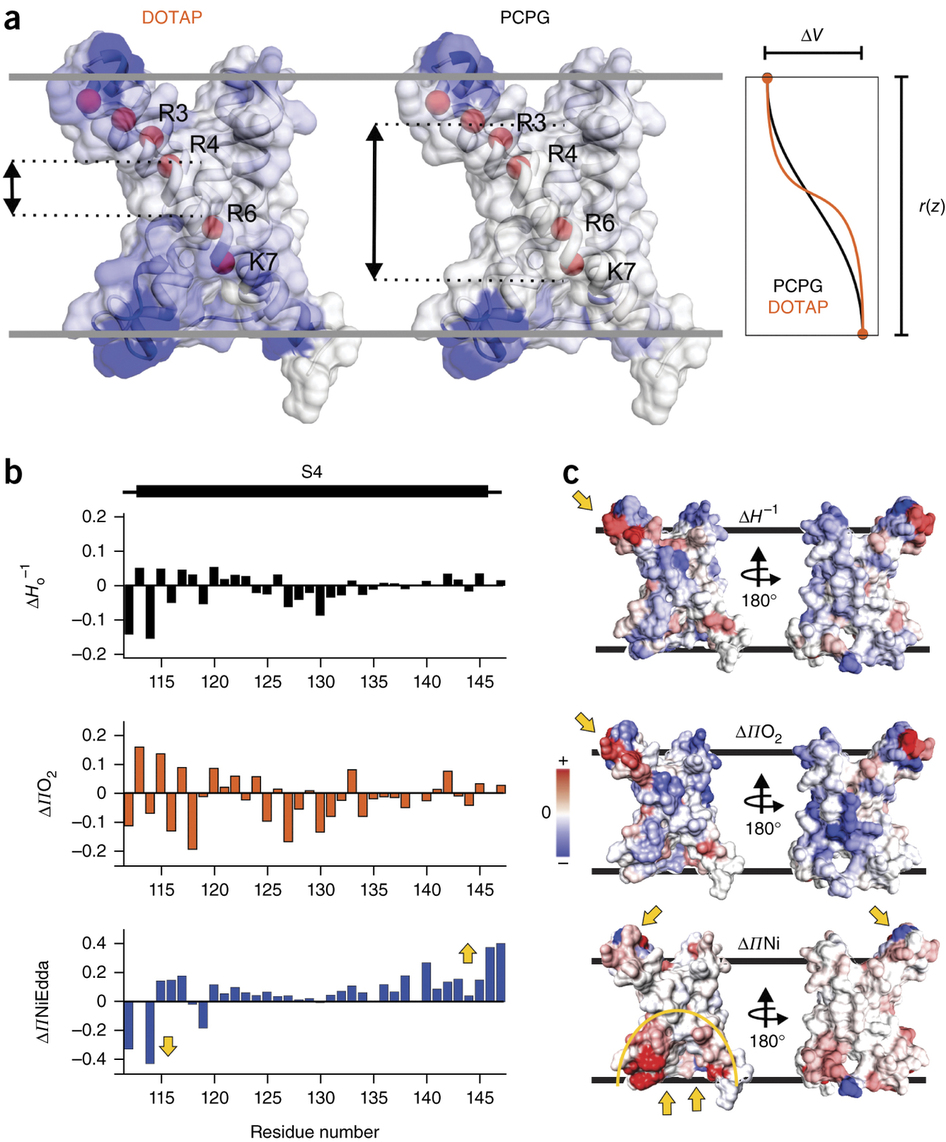
Voltage-gated ion channels respond to transmembrane electric fields through reorientations of the positively charged S4 helix within the voltage-sensing domain (VSD). Despite a wealth of structural and functional data, the details of this conformational change remain controversial. Recent electrophysiological evidence showed that equilibrium between the resting (‘down’) and activated (‘up’) conformations of the KvAP VSD from Aeropyrum pernix can be biased through reconstitution in lipids with or without phosphate groups. We investigated the structural transition between these functional states, using site-directed spin-labeling and EPR spectroscopic methods. Solvent accessibility and interhelical distance determinations suggest that KvAP gates through S4 movements involving an ∼3-Å upward tilt and simultaneous ∼2-Å axial shift. This motion leads to large accessibly changes in the intracellular water-filled crevice and supports a new model of gating that combines structural rearrangements and electric-field remodeling.