Structure of the Get3 targeting factor in complex with its membrane protein cargo
By Agnieszka Mateja, Marcin Paduch, Hsin-Yang Chang, Anna Szydlowska, Anthony A. Kossiakoff, Ramanujan S. Hegde, and Robert J. Keenan.
Published in Science. 2015 Mar 6;347(6226):1152-5. PMID: 25745174. PMCID: 4413028. Link to publication page.
Core Facility: Synthetic Antigen Binder (SAB) Generation and Crystallography
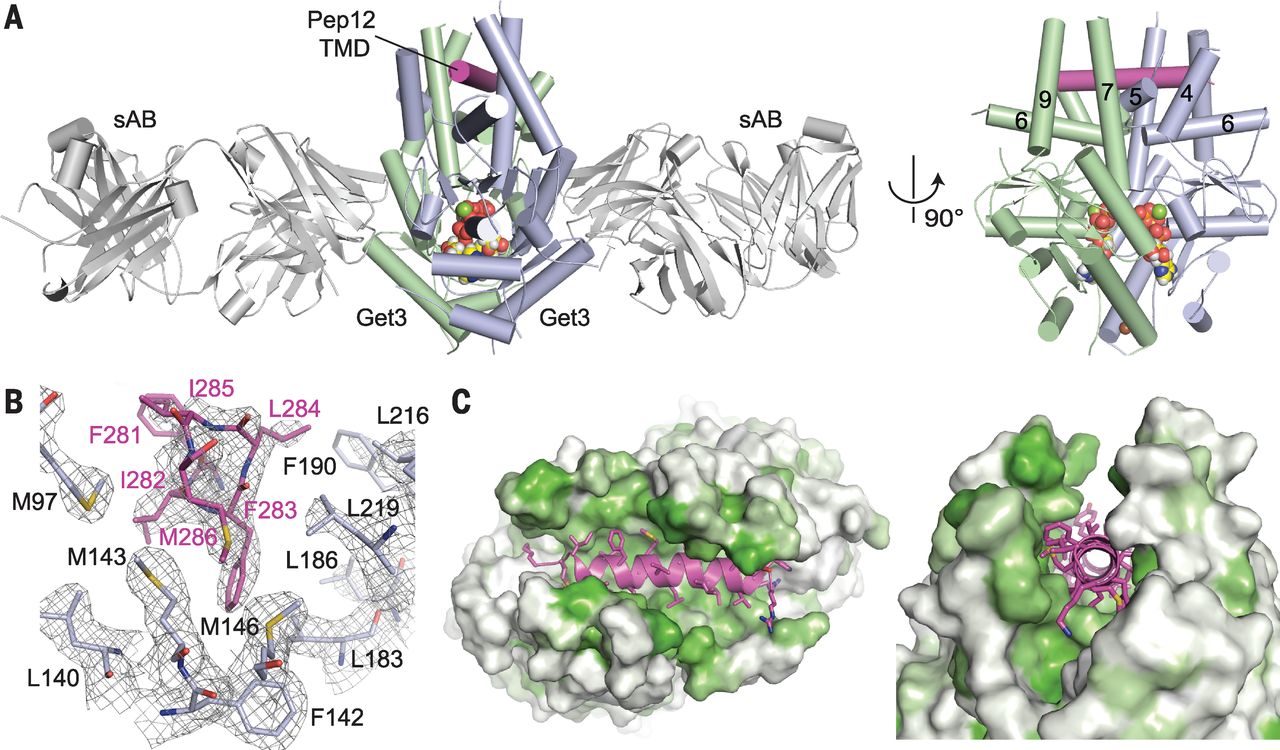
Figure 2. The helical TMD of a TA substrate binds deep within the composite hydrophobic groove of dimeric Get3. (A) Overview of dimeric Saccharomyces cerevisiae Get3 bound to a truncated Pep12 TA substrate (magenta) and nucleotide (spheres), and sandwiched between two copies of an engineered sAB (gray). At right, a “side” view of the complex is shown with sABs removed for clarity. (B) Details of the interaction between the Pep12 TMD C terminus and a methionine-rich cluster at one end of the hydrophobic groove. Electron density is from a 2.05 Å 2Fo – Fc map contoured at 1.0σ. (Amino acid abbreviations: F, Phe; I, Ile; L, Leu; and M, Met.) (C) Surface representations of the TA substrate-binding site, colored from least (white) to most (green) hydrophobic.
Abstract
Tail-anchored (TA) proteins are a physiologically important class of membrane proteins targeted to the endoplasmic reticulum by the conserved guided-entry of TA proteins (GET) pathway. During transit, their hydrophobic transmembrane domains (TMDs) are chaperoned by the cytosolic targeting factor Get3, but the molecular nature of the functional Get3-TA protein targeting complex remains unknown. We reconstituted the physiologic assembly pathway for a functional targeting complex and showed that it comprises a TA protein bound to a Get3 homodimer. Crystal structures of Get3 bound to different TA proteins showed an α-helical TMD occupying a hydrophobic groove that spans the Get3 homodimer. Our data elucidate the mechanism of TA protein recognition and shielding by Get3 and suggest general principles of hydrophobic domain chaperoning by cellular targeting factors.
Editor’s Summary
How to GET to the right membrane
Membrane proteins with a hydrophobic transmembrane domain (TMD) play critical roles in virtually all aspects of cell physiology. After it has been synthesized in the cytosol, this TMD must be targeted to and inserted into the correct membrane. The GET pathway is one of two targeting pathways to the endoplasmic reticulum conserved across all eukaryotes. It is not clear how the central targeting factor, Get3, recognizes a TMD to shield it from aggregation until it is successfully inserted into the membrane. Now, Mateja et al. show that the functional targeting complex comprises a Get3 dimer bound to a single TMD. The helical hydrophobic TMD binds deep within a large hydrophobic groove in the Get3 dimer. This groove closes slightly upon TMD binding, forming a dynamic “lid” over the mouth of the groove.