Symmetry-constrained Analysis of Pulsed Double Electron-Electron Resonance (DEER) Spectroscopy Reveals the Dynamic Nature of the KcsA Activation Gate
By Olivier Dalmas, H. Clark Hyde, Raymond E. Hulse, and Eduardo Perozo.
Published in J Am Chem Soc on 2012 Oct 3;134(39):16360-9. PMID: 22946877. PMCID: PMC3845908. Link to publication page.
Project: Dynamics of Ion Permeation and Conformational Coupling in – KcsA
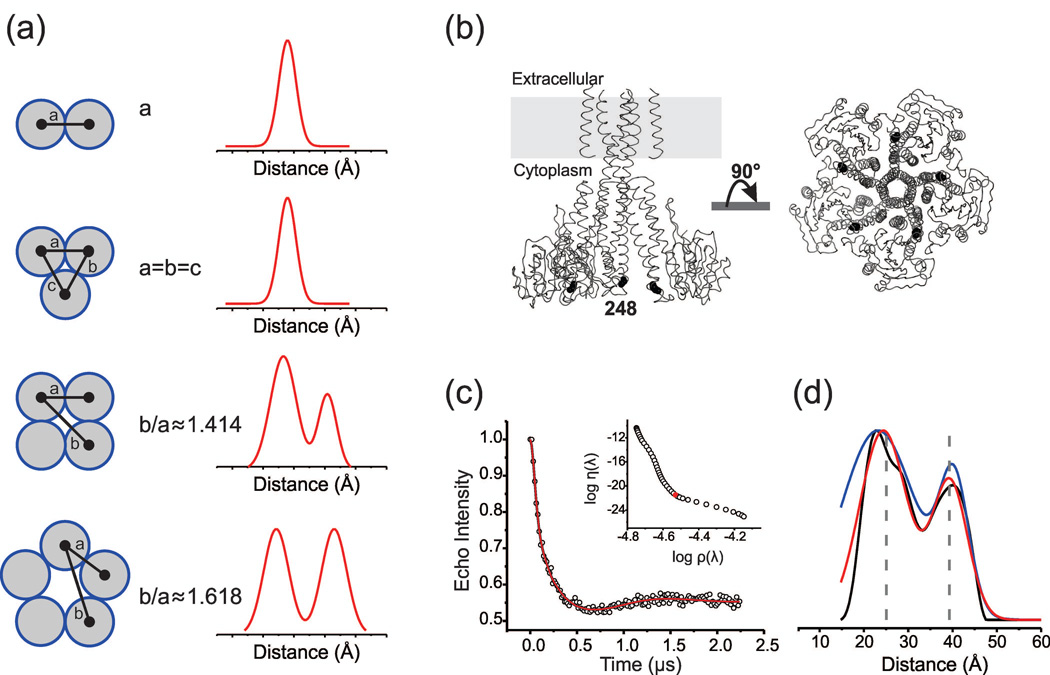
Figure 1. Distance determination in multimeric proteins. (a) Schematic representation of the expected distance distribution profile as a function of the oligomeric state of a homomeric protein. (b) Cysteine mutation in CorA is shown at position V248 as black spheres at Cβ. For clarity, only 3 subunits are shown. (c) The DEER refocused echo intensity is plotted (open circles) vs. the evolution time. Fits determined from Tikhonov regularization, 2 Rice3D and symmetry-constrained 2 Rice3D models are shown in black, blue, and red, respectively. In the inset, the optimal regularization parameter (λ=158) is shown at the corner of the L-curve. (d) Corresponding distance distributions are plotted using the same color code. The vertical dashed lines represent the average spin-spin distances calculated from rotameric libraries of the spin label conjugated to the V248C label site.
Abstract
Distance determination from an echo intensity modulation obtained by pulsed double electron-electron resonance (DEER) experiment is a mathematically ill-posed problem. Tikhonov regularization yields distance distributions that can be difficult to interpret, especially in system with multiple discrete distance distributions. Here, we show that by using geometric fit constraints in symmetric homo-oligomeric protein systems, we were able to increase the accuracy of a model-based fit solution based on a sum of Gaussian distributions. Our approach was validated on two different ion channels of known oligomeric states, KcsA (a tetramer) and CorA (a pentamer). Statistical analysis of the resulting fits was integrated within our method to help the experimenter evaluate the significance of a symmetry-constrained vs. standard model distribution fit and to examine multi-distance confidence regions. This approach was used to quantitatively evaluate the role of the C-terminal domain (CTD) on the flexibility and conformation of the activation gate of the K+ channel KcsA. Our analysis reveals a significant increase in the dynamics of the inner bundle gate upon opening. Also, it explicitly demonstrates the degree to which the CTD restricts the motion of the lower gate at rest and during activation gating.


