The cost of living in the membrane: A case study of hydrophobic mismatch for the multi-segment protein LeuT
By Sayan Mondal, George Khelashvili, Lei Shi, and Harel Weinstein.
Published in Chemistry and Physics of Lipids 169: 27–38 on January 30, 2013. PMID: 23376428. PMCID: PMC3631462. Link to publication page.
Project: The Transport Cycle in Neurotransmitter Uptake Systems. Core Facility: Computational Modeling.
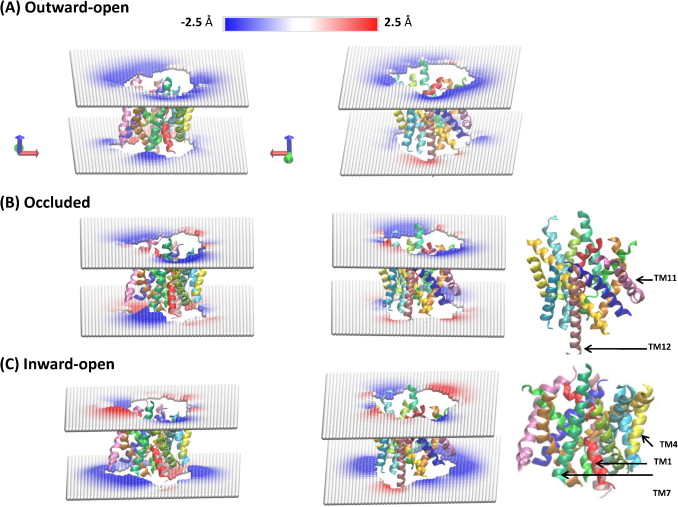
Figure 2. Local deformations of the upper and lower leaflets of the POPC lipid bilayer around LeuT in its (A) outward-open, (B) occluded, and (C) inward-open conformations. The membrane deformation profiles for each conformation are shown from two different angles (right and left columns), and the TM-bundle of the protein is shown to provide a frame of reference for the membrane deformations. The deformations were calculated for each leaflet with the CTMD approach on a (100 Å × 100 Å) grid with a spacing of 2 Å. Only regions of the bilayer with lipids in both leaflets were considered in the continuum calculations, effectively removing phospholipids interacting with the protein as individual molecules.
Abstract
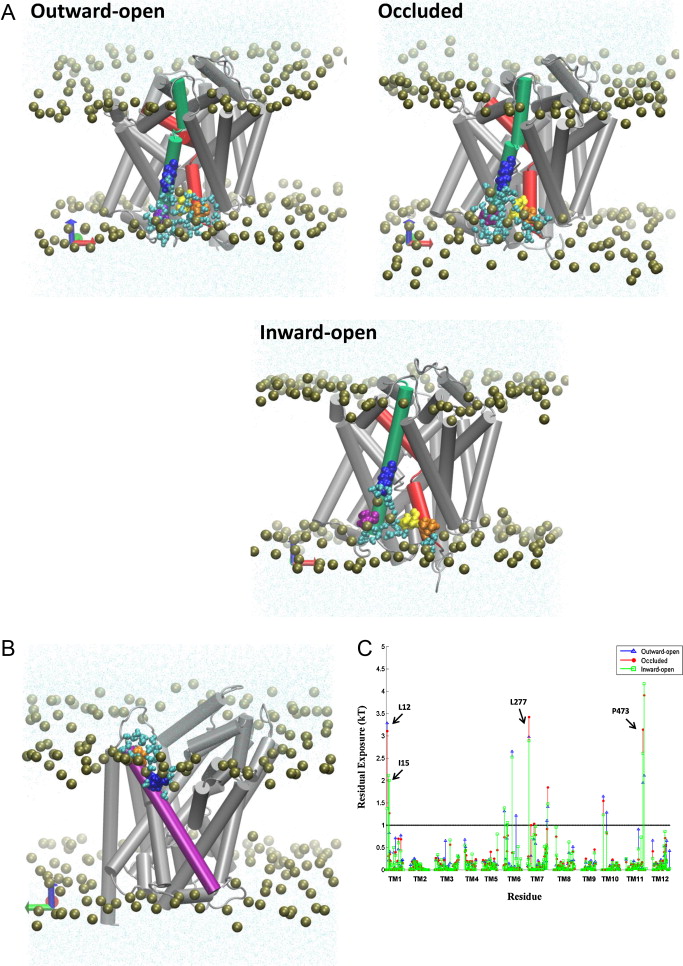
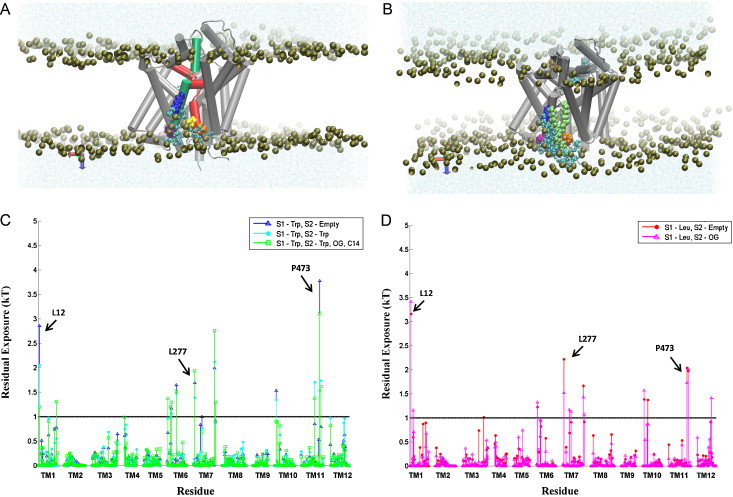
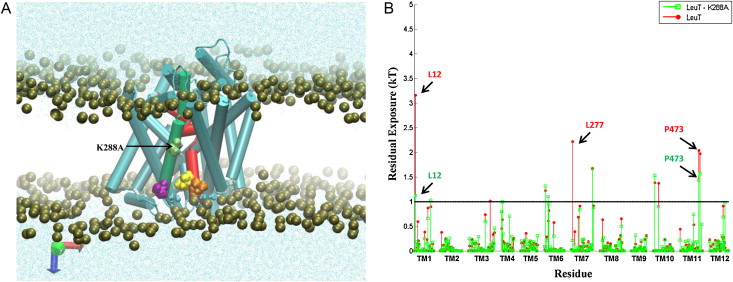
Many observations of the role of the membrane in the function and organization of transmembrane (TM) proteins have been explained in terms of hydrophobic mismatch between the membrane and the inserted protein. For a quantitative investigation of this mechanism in the lipid-protein interactions of functionally relevant conformations adopted by a multi-TM segment protein, the bacterial leucine transporter (LeuT), we employed a novel method, Continuum-Molecular Dynamics (CTMD), that quantifies the energetics of hydrophobic mismatch by combining the elastic continuum theory of membrane deformations with an atomistic level description of the radially asymmetric membrane-protein interface from MD simulations. LeuT has been serving as a model for structure-function studies of the mammalian neurotransmitter:sodium symporters (NSSs), such as the dopamine and serotonin transporters, which are the subject of intense research in the field of neurotransmission. The membrane models in which LeuT was embedded for these studies were composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) lipid, or 3:1 mixture of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) lipids. The results show that deformation of the host membrane alone is not sufficient to alleviate the hydrophobic mismatch at specific residues of LeuT. The calculations reveal significant membrane thinning and water penetration due to the specific local polar environment produced by the charged K288 of TM7 in LeuT, that is membrane-facing deep inside the hydrophobic milieu of the membrane. This significant perturbation is shown to result in unfavorable polar-hydrophobic interactions at neighboring hydrophobic residues in TM1a and TM7. We show that all the effects attributed to the K288 residue (membrane thinning, water penetration, and the unfavorable polar-hydrophobic interactions at TM1a and TM7), are abolished in calculations with the K288A mutant. The involvement of hydrophobic mismatch is somewhat different in the functionally distinct conformations (outward-open, occluded, inward-open) of LeuT, and the differences are shown to connect to structural elements (e.g., TM1a) known to play key roles in transport. This finding suggests a mechanistic hypothesis for the enhanced transport activity observed for the K288A mutant, suggesting that the unfavorable hydrophobic-hydrophilic interactions hinder the motion of TM1a in the functionally relevant conformational transition to the inward-open state. Various extents of such unfavorable interactions, involving exposure to the lipid environment of adjacent hydrophobic and polar residues, are common in multi-segment transmembrane proteins, and must be considered to affect functionally relevant conformational transitions.