The hitchhiker’s guide to the voltage-gated sodium channel galaxy
By Christopher A Ahern, Jian Payandeh, Frank Bosmans, and Baron Chanda.
Published in J Gen Physiol 2016 Jan;147(1):1-24.
PMID: 26712848. PMCID: PMC4692491. Link to Pubmed page.
Core Facility: Membrane Protein Expression/Purification.
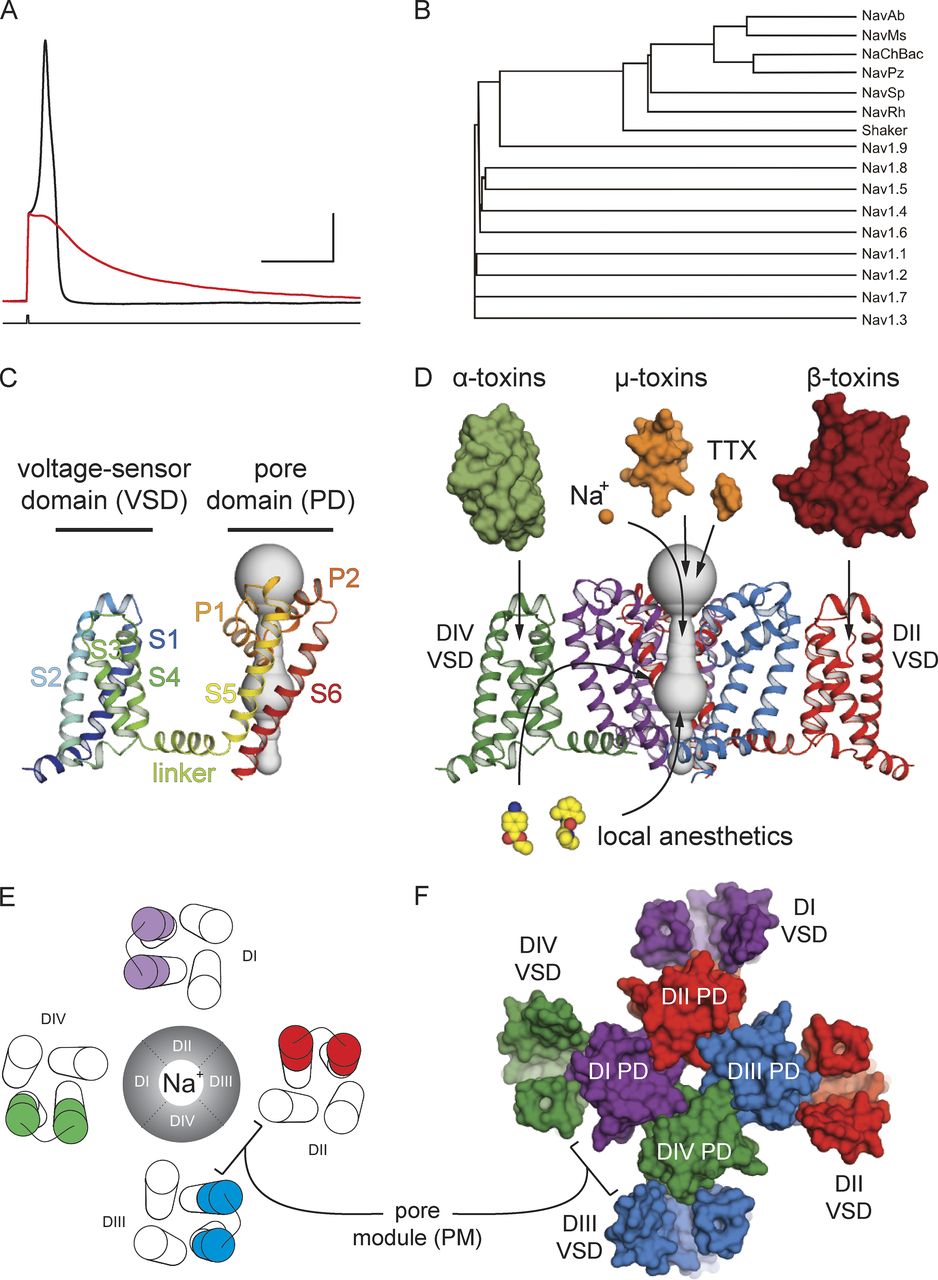
Figure 1. Nav channel function, family tree, and structural architecture. (A) Evoked action potential recorded from a mouse DRG neuron at room temperature before (black) and after (red) the application of 1 µM TTX. X axis is 30 ms, and y axis is 20 mV. (B) A phylogenetic tree of Nav channels as well as Shaker obtained using Vector NTI AlignX software. (C) The side view of a signal subunit of the NavAb channel homotetramer (Protein Data Bank accession no. 3RVY) in ribbon style is colored from N terminus (blue) to C terminus (red). This view highlights the VSD as a modular four-helix bundle. (D) Side view of the NavAb channel with the front VSD and pore domain removed for clarity. For illustrative purposes, NavAb is colored according to a pseudotetrameric arrangement expected for eukaryotic Nav cannels. Representative classes of protein toxins (α, β, and µ), small molecule toxins (TTX), as well select small molecule drugs (lidocaine and benzocaine) are represented with arrows pointing to their presumed canonical binding sites on the channel. (E) Top-view schematic of a eukaryotic Nav channel with the S3b–S4 region of the VSDs from different domains is highlighted in different colors. The ion-conducting Na+ pore is found in the center of this view. (F) A structural top view of the NavAb channel colored according to a pseudotetrameric arrangement expected for a eukaryotic Nav channel (as in D). This subunit coloring highlights the “domain-swapped arrangement” of the VSDs around the PM observed for all voltage-gated ion channels.
Abstract
Eukaryotic voltage-gated sodium (Nav) channels contribute to the rising phase of action potentials and served as an early muse for biophysicists laying the foundation for our current understanding of electrical signaling. Given their central role in electrical excitability, it is not surprising that (a) inherited mutations in genes encoding for Nav channels and their accessory subunits have been linked to excitability disorders in brain, muscle, and heart; and (b) Nav channels are targeted by various drugs and naturally occurring toxins. Although the overall architecture and behavior of these channels are likely to be similar to the more well-studied voltage-gated potassium channels, eukaryotic Nav channels lack structural and functional symmetry, a notable difference that has implications for gating and selectivity. Activation of voltage-sensing modules of the first three domains in Nav channels is sufficient to open the channel pore, whereas movement of the domain IV voltage sensor is correlated with inactivation. Also, structure-function studies of eukaryotic Nav channels show that a set of amino acids in the selectivity filter, referred to as DEKA locus, is essential for Na(+) selectivity. Structures of prokaryotic Nav channels have also shed new light on mechanisms of drug block. These structures exhibit lateral fenestrations that are large enough to allow drugs or lipophilic molecules to gain access into the inner vestibule, suggesting that this might be the passage for drug entry into a closed channel. In this Review, we will synthesize our current understanding of Nav channel gating mechanisms, ion selectivity and permeation, and modulation by therapeutics and toxins in light of the new structures of the prokaryotic Nav channels that, for the time being, serve as structural models of their eukaryotic counterparts.


