Transport domain unlocking sets the uptake rate of an aspartate transporter
By Nurunisa Akyuz, Elka R. Georgieva, Zhou Zhou, Sebastian Stolzenberg, Michel A. Cuendet, George Khelashvili, Roger B. Altman, Daniel S. Terry, Jack H. Freed, Harel Weinstein, Olga Boudker, and Scott C. Blanchard.
Published in Nature. 2015 Feb 5;518(7537):68-73. PMID: 25652997. PMCID: PMC4351760. [Available on 2015-08-05] Link to publication page.
Projects: The Transport Cycle in Neurotransmitter Uptake Systems, Conformational Dynamics of a Glutamate Transporter Homologue | Core Facility: Computational Modeling
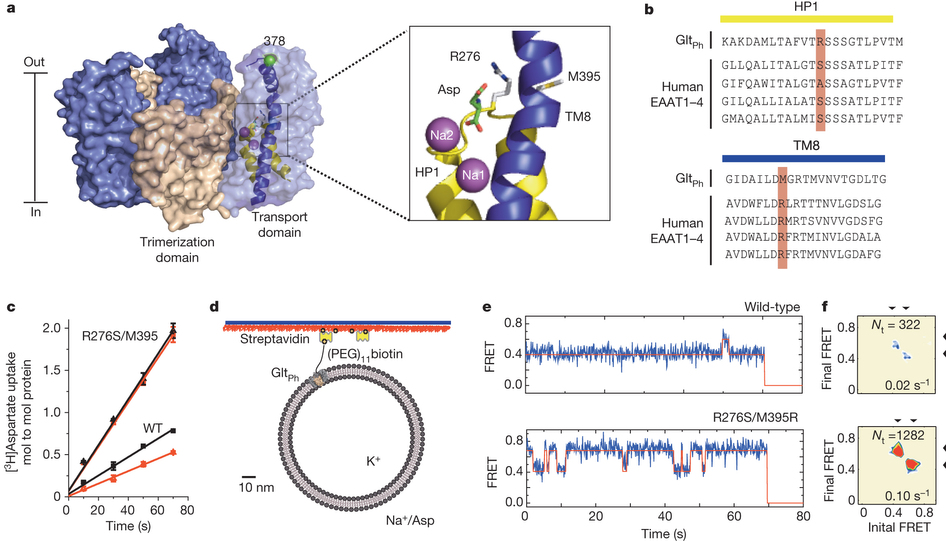
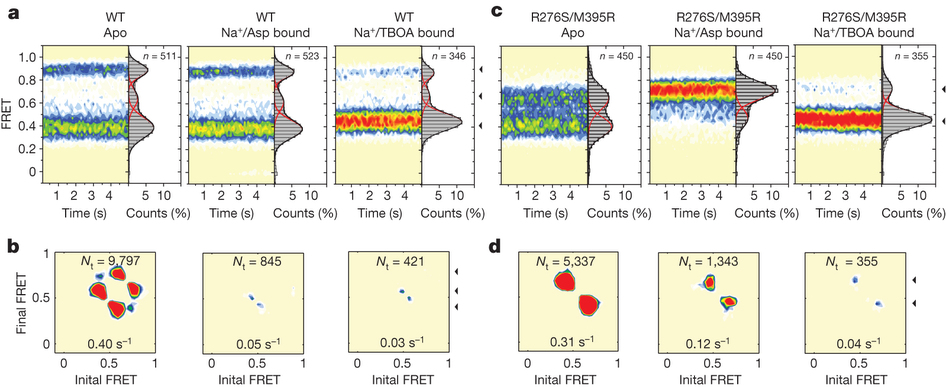
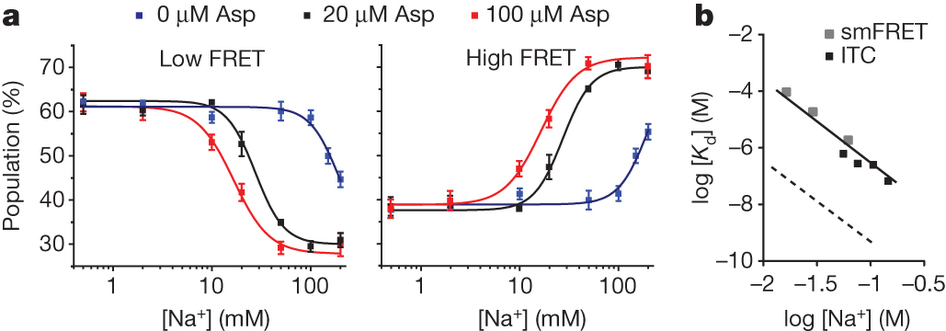
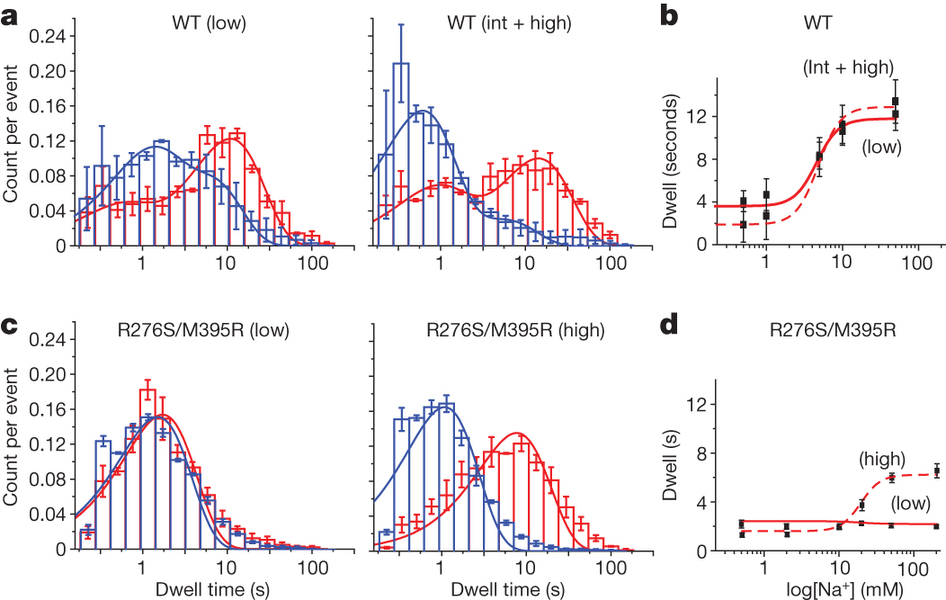
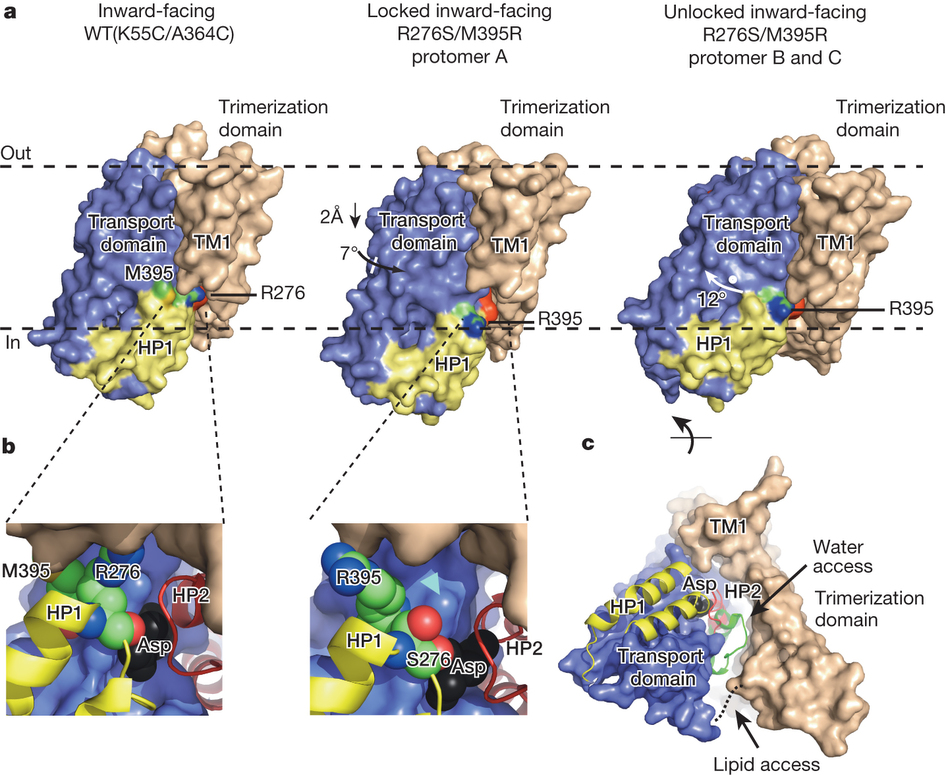
Figure 1. Transport rates and ‘elevator-like’ domain dynamics are correlated. a, Surface representation of the outward-facing GltPh showing the transport (blue) and scaffold (beige) domains. In one protomer, HP1 (yellow) and TM8 (dark blue) are emphasized as cartoons. In the enlarged substrate-binding site (right), mutated residues and aspartate are shown as sticks and ions are shown as spheres. b, Sequence alignment for HP1 and TM8 with mutation sites highlighted in pink. c, Aspartate uptake by unlabelled (black) and labelled (red) transporters. Substrate uptake data are shown as averages of at least three experiments with error bars representing standard deviations. d, Proteoliposome attachment strategy. e, smFRET trajectories recorded for the wild type (WT) and mutant in proteoliposomes under transport conditions. f, Transition density plots corresponding to e. The average transition frequencies and the number of total transitions (Nt) are shown. Colour scale is from tan (lowest) to red (highest frequency).
Abstract
Glutamate transporters terminate neurotransmission by clearing synaptically released glutamate from the extracellular space, allowing repeated rounds of signalling and preventing glutamate-mediated excitotoxicity. Crystallographic studies of a glutamate transporter homologue from the archaeon Pyrococcus horikoshii, GltPh, showed that distinct transport domains translocate substrates into the cytoplasm by moving across the membrane within a central trimerization scaffold. Here we report direct observations of these ‘elevator-like’ transport domain motions in the context of reconstituted proteoliposomes and physiological ion gradients using single-molecule fluorescence resonance energy transfer (smFRET) imaging. We show that GltPh bearing two mutations introduced to impart characteristics of the human transporter exhibits markedly increased transport domain dynamics, which parallels an increased rate of substrate transport, thereby establishing a direct temporal relationship between transport domain motion and substrate uptake. Crystallographic and computational investigations corroborated these findings by revealing that the ‘humanizing’ mutations favour structurally ‘unlocked’ intermediate states in the transport cycle exhibiting increased solvent occupancy at the interface between the transport domain and the trimeric scaffold.