Unfolding of a Temperature-Sensitive Domain Controls Voltage-Gated Channel Activation
By Cristina Arrigoni, Ahmed Rohaim, David Shaya, Felix Findeisen, Richard A. Stein, Shailika Reddy Nurva, Smriti Mishra, Hassane S Mchaourab, and Daniel L. Minor.
Published in Cell. 2016 Feb 25;164(5):922-36. PMID: 26919429. PMCID: PMC4769381. Link to Pubmed page.
Core Facility: Spectroscopy and Instrumentation.
Abstract
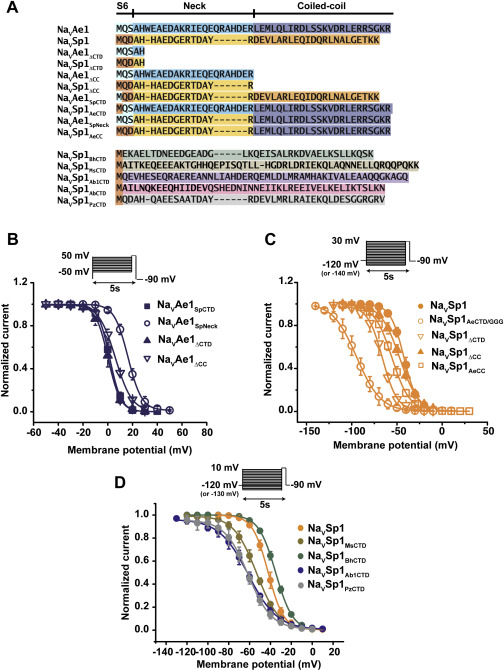
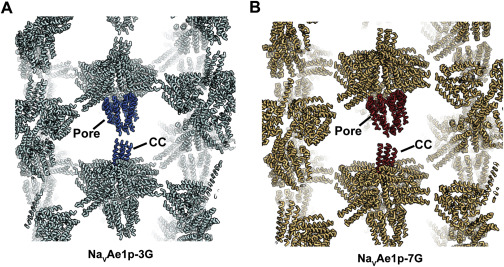
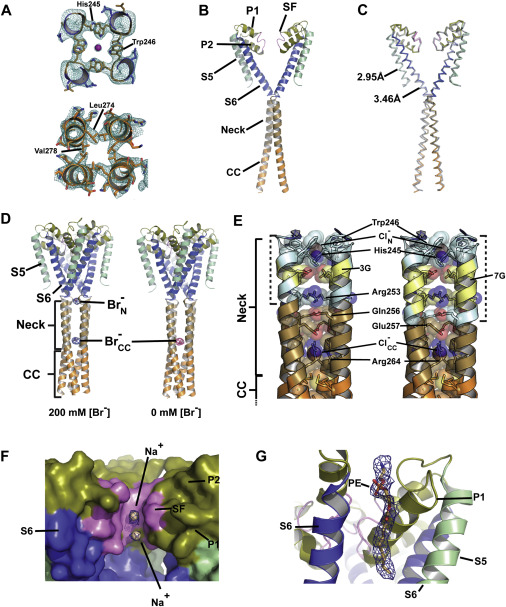
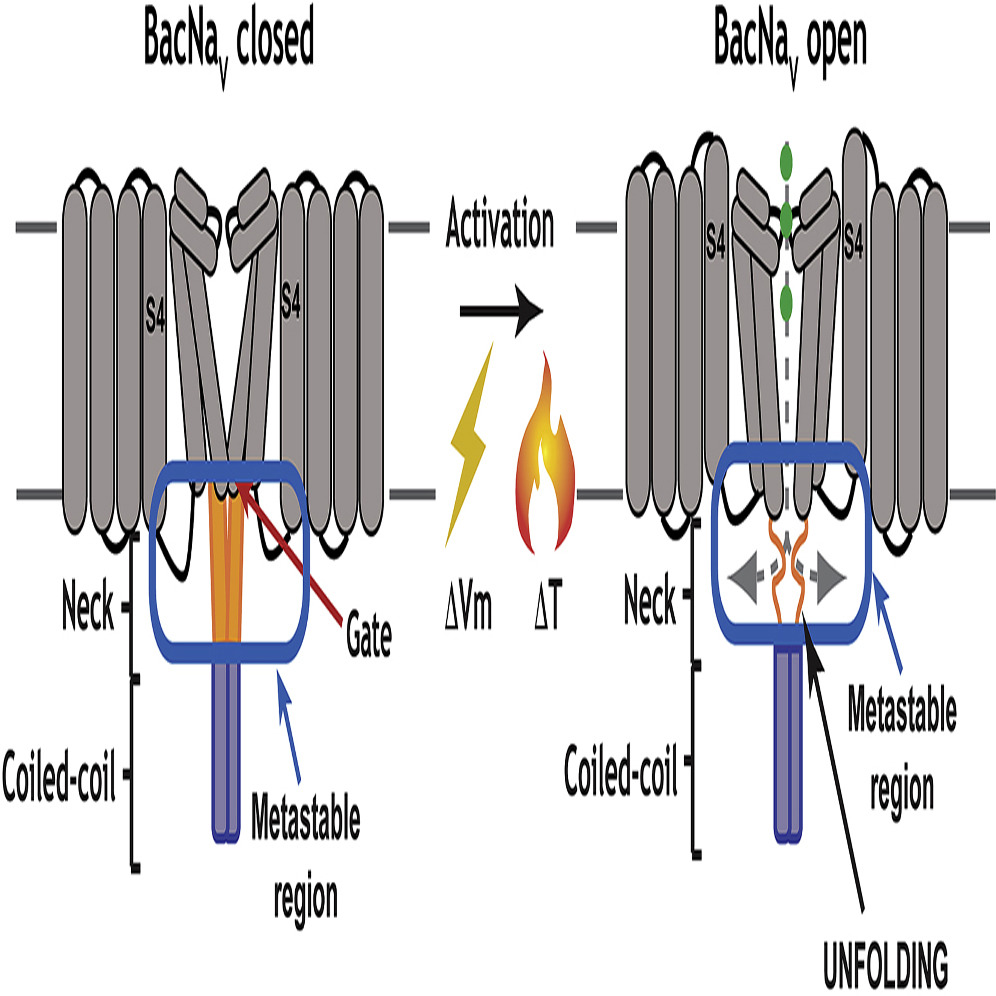
Voltage-gated ion channels (VGICs) are outfitted with diverse cytoplasmic domains that impact function. To examine how such elements may affect VGIC behavior, we addressed how the bacterial voltage-gated sodium channel (BacNaV) C-terminal cytoplasmic domain (CTD) affects function. Our studies show that the BacNaV CTD exerts a profound influence on gating through a temperature-dependent unfolding transition in a discrete cytoplasmic domain, the neck domain, proximal to the pore. Structural and functional studies establish that the BacNaV CTD comprises a bi-partite four-helix bundle that bears an unusual hydrophilic core whose integrity is central to the unfolding mechanism and that couples directly to the channel activation gate. Together, our findings define a general principle for how the widespread four-helix bundle cytoplasmic domain architecture can control VGIC responses, uncover a mechanism underlying the diverse BacNaV voltage dependencies, and demonstrate that a discrete domain can encode the temperature-dependent response of a channel.