Water access points and hydration pathways in CLC H+/Cl- transporters
By Wei Han, Ricky C. Cheng, Merritt C. Maduke, and Emad Tajkhorshid.
Published in Proceedings of the National Academy of Sciences of the United States of America 2014 Feb 4;111(5):1819-24. PMID: 24379362. PMID: 24379362. PMCID: PMCID3918786 Link to publication page.
Projects: The Transport Cycle in Neurotransmitter Uptake Systems, Conformational Dynamics in the CLC Channel/Transporter Family . Core Facility: Computational Modeling.
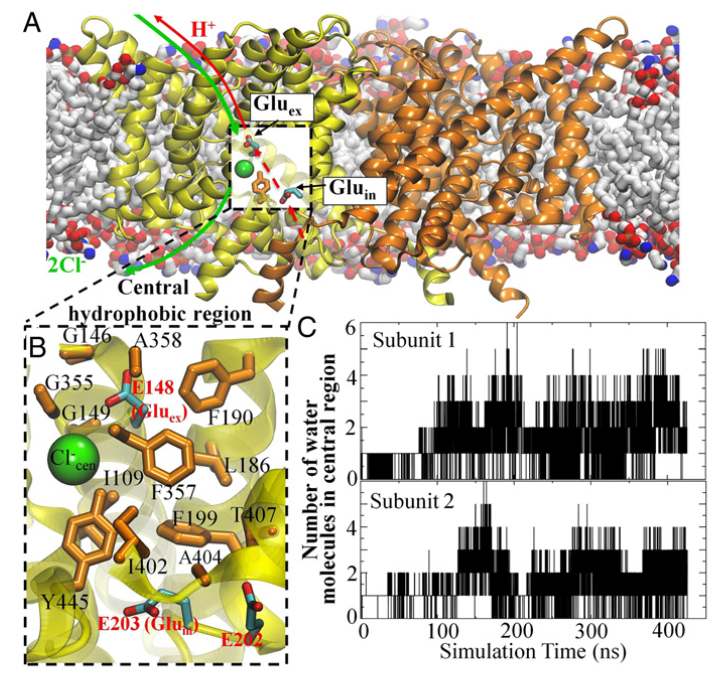
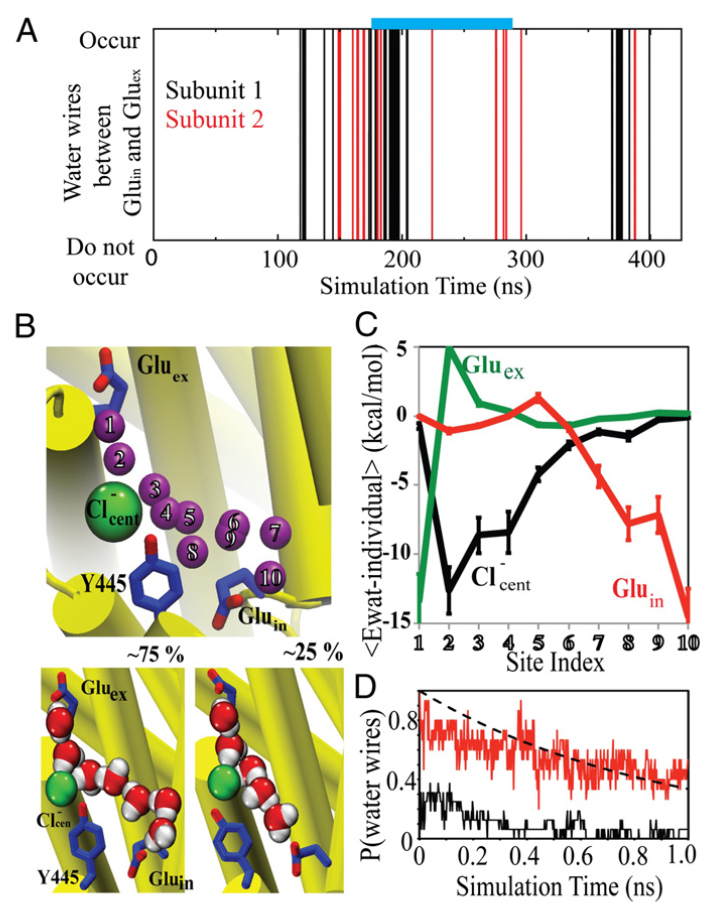
Figure 1. Cl− and H+ permeation pathways in ClC-ec1. (A) View of the ClC-ec1 structure in a lipid bilayer (the simulation system used here), with the identical subunits shown in yellow and orange. The presumed Cl−/H+ permeation pathways are indicated by green and red lines, respectively. The dashed segment of the red line denotes the pathway investigated in this study. (B) Close-up of the central hydrophobic region, with the residues forming this region shown as orange sticks and labeled. Also shown are key glutamate residues (E202, E203, and E148) as well as the Cl− at the central anion binding site. (C) Hydration of the central hydrophobic region during the 0.4-μs equilibrium simulation, measured as the number of water molecules in this region for each subunit.
Significance
CLC transporters are biologically essential proteins that catalyze the transmembrane exchange of chloride for protons. The permeation pathway for chloride through the transporters has been well characterized. Here, we study the more elusive permeation pathway for protons. Through computational modeling, we show that water molecules can permeate deep inside the protein and form continuous wires. To test the hypothesis that these water wires mediate proton transport, we mutated residues predicted to impede water wire formation and experimentally evaluated the effects of the mutations. The results from our concerted computational and experimental approach strongly support the role of water in proton transport by CLCs and provide a valuable framework for investigating their overall transport mechanism.
Abstract
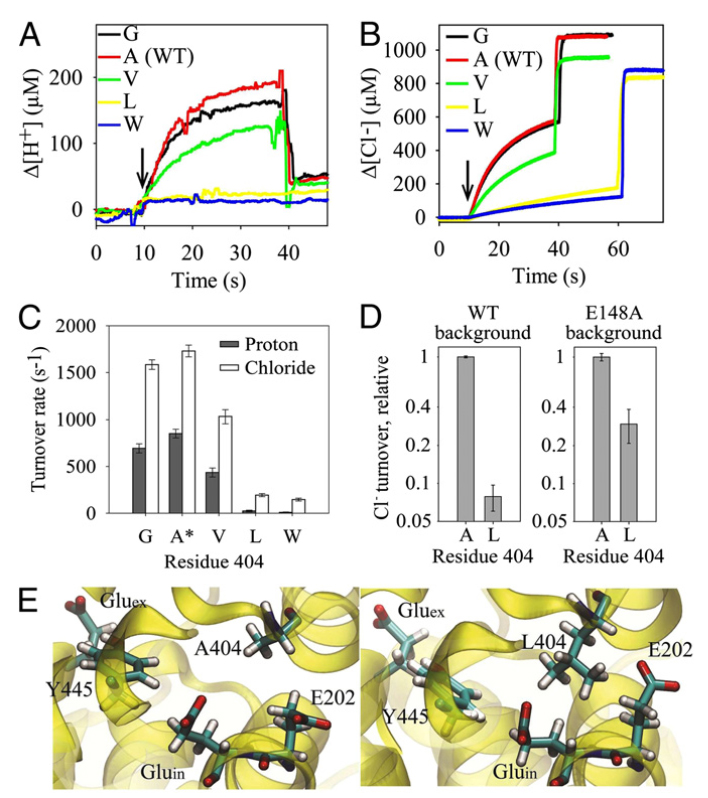
CLC transporters catalyze transmembrane exchange of chloride for protons. Although a putative pathway for Cl− has been established, the pathway of H+ translocation remains obscure. Through a highly concerted computational and experimental approach, we characterize microscopic details essential to understanding H+-translocation. An extended (0.4 µs) equilibrium molecular dynamics simulation of membrane-embedded, dimeric ClC-ec1, a CLC from Escherichia coli, reveals transient but frequent hydration of the central hydrophobic region by water molecules from the intracellular bulk phase via the interface between the two subunits. We characterize a portal region lined by E202, E203, and A404 as the main gateway for hydration. Supporting this mechanism, site-specific mutagenesis experiments show that ClC-ec1 ion transport rates decrease as the size of the portal residue at position 404 is increased. Beyond the portal, water wires form spontaneously and repeatedly to span the 15-Å hydrophobic region between the two known H+ transport sites [E148 (Gluex) and E203 (Gluin)]. Our finding that the formation of these water wires requires the presence of Cl− explains the previously mystifying fact that Cl− occupancy correlates with the ability to transport protons. To further validate the idea that these water wires are central to the H+ transport mechanism, we identified I109 as the residue that exhibits the greatest conformational coupling to water wire formation and experimentally tested the effects of mutating this residue. The results, by providing a detailed microscopic view of the dynamics of water wire formation and confirming the involvement of specific protein residues, offer a mechanism for the coupled transport of H+ and Cl− ions in CLC transporters.