Conformational Transitions and Alternating-Access Mechanism in the Sarcoplasmic Reticulum Calcium Pump
By Avisek Das, Huan Rui, Robert Nakamoto, Benoît Roux.
Published in J Mol Biol. 2017 Mar 10;429(5):647-666. PMID: 28093226. Link to Pubmed page.
Project: Dynamics of Ion Permeation and Conformational Coupling in – KcsA. Core Facility: Computational Modeling. Membrane Protein Production and Chemistry.
Abstract
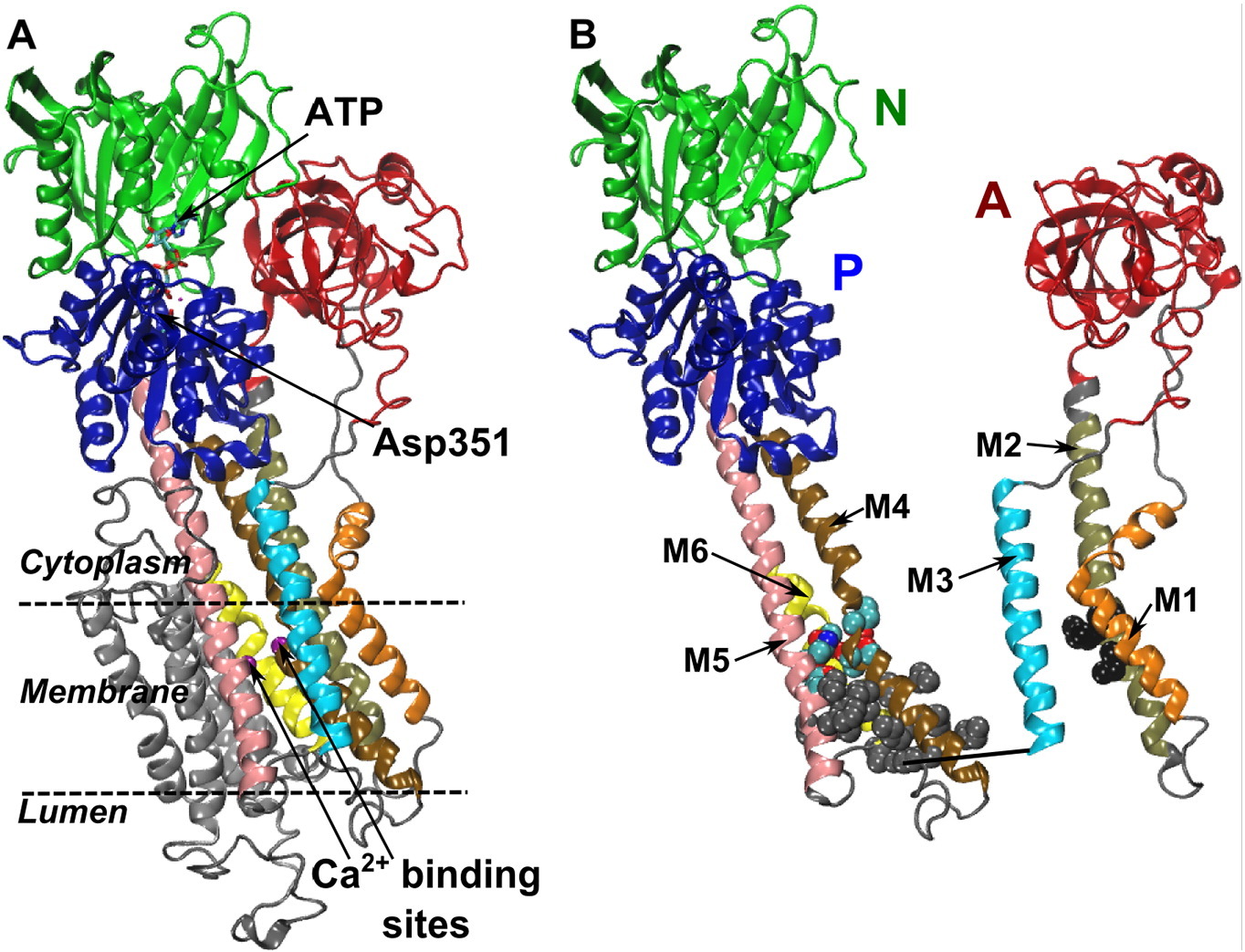
Ion pumps are integral membrane proteins responsible for transporting ions against concentration gradients across biological membranes. Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), a member of the P-type ATPases family, transports two calcium ions per hydrolyzed ATP molecule via an “alternating-access” mechanism. High-resolution crystallographic structures provide invaluable insight on the structural mechanism of the ion pumping process. However, to understand the molecular details of how ATP hydrolysis is coupled to calcium transport, it is necessary to gain knowledge about the conformational transition pathways connecting the crystallographically resolved conformations. Large-scale transitions in SERCA occur at time-scales beyond the current reach of unbiased molecular dynamics simulations. Here, we overcome this challenge by employing the string method, which represents a transition pathway as a chainofstates linking two conformational endpoints. Using a multiscale methodology, we have determined all-atom transition pathways for three main conformational transitions responsible for the alternating-access mechanism. The present pathways provide a clear chronology and ordering of the key events underlying the active transport of calcium ions by SERCA. Important conclusions are that the conformational transition that leads to occlusion with bound ATP and calcium is highly concerted and cooperative, the phosphorylation of Asp351 causes areorganization of the cytoplasmic domains that subsequently drives the opening of the luminal gate, and thereclosing of luminal gate induces a shift in the cytoplasmic domains that subsequently enables the dephosphorylation of Asp351-P. Formation of transient residue-residue contacts along the conformational transitions predicted by the computations provide an experimental route to test the general validity of the computational pathways.


