Energy transduction and alternating access of the mammalian ABC transporter P-glycoprotein
By Brandy Verhalen, Reza Dastvan, Sundarapandian Thangapandian, Yelena Peskova, Hanane A. Koteiche, Robert Nakamoto, Emad Tajkhorshid, and Hassane Mchaourab.
Published in Nature. 2017 Mar 30;543(7647):738-741. PMID: 28289287. Link to Pubmed page.
Project: Structural Dynamics of ABC Transporter. Core Facility: Computational Modeling. Membrane Protein Production and Chemistry
.
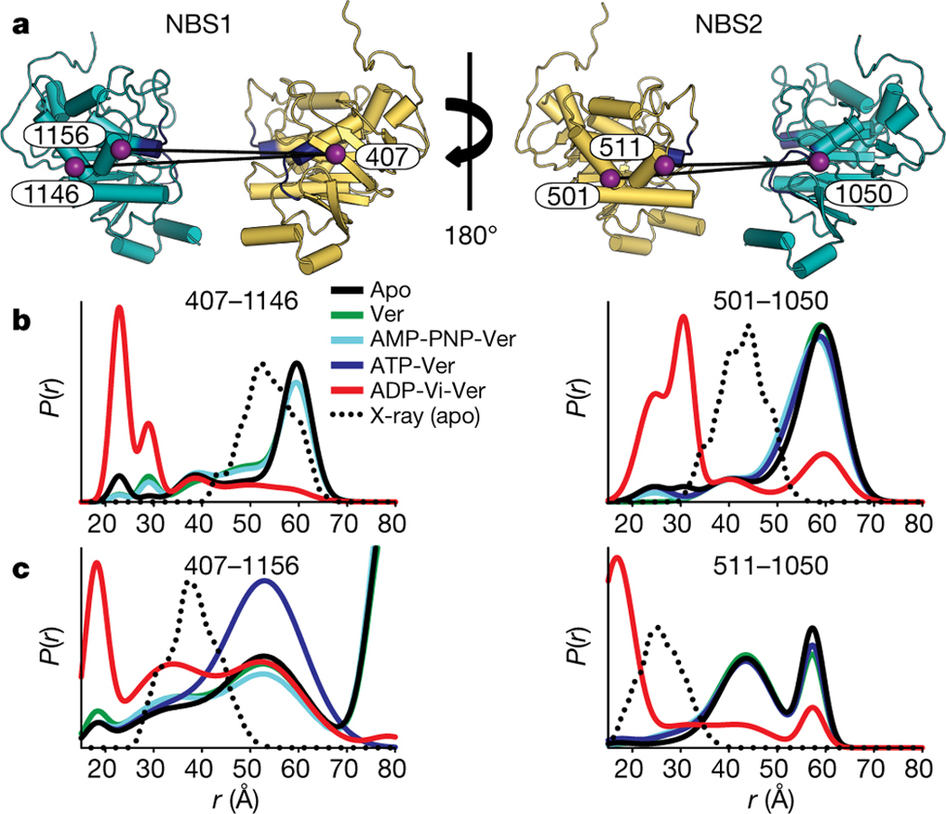
Figure 2. a, Cartoon representation of the NBSs viewed from the side. Each NBS was monitored by two spin label pairs introduced at structurally equivalent sites. Conserved Walker A and ABC signature motifs are highlighted in dark blue. b, c, These spin label pairs report distance changes expected from the formation of the NBD dimer in the transition state.
Abstract
ATP binding cassette (ABC) transporters of the exporter class harness the energy of ATP hydrolysis in the nucleotide-binding domains (NBDs) to power the energetically uphill efflux of substrates by a dedicated transmembrane domain (TMD). Although numerous investigations have described the mechanism of ATP hydrolysis and defined the architecture of ABC exporters, a detailed structural dynamic understanding of the transduction of ATP energy to the work of substrate translocation remains elusive. Here we used double electron-electron resonance and molecular dynamics simulations to describe the ATP- and substrate-coupled conformational cycle of the mouse ABC efflux transporter P-glycoprotein (Pgp; also known as ABCB1), which has a central role in the clearance of xenobiotics and in cancer resistance to chemotherapy. Pairs of spin labels were introduced at residues selected to track the putative inward-facing to outward-facing transition. Our findings illuminate how ATP energy is harnessed in the NBDs in a two-stroke cycle and elucidate the consequent conformational motion that reconfigures the TMD, two critical aspects of Pgp transport mechanism. Along with a fully atomistic model of the outward-facing conformation in membranes, the insight into Pgp conformational dynamics harmonizes mechanistic and structural data into a novel perspective on ATP-coupled transport and reveals mechanistic divergence within the efflux class of ABC transporters.


