Drs. Shohei Koide and Anthony Kossiakoff recently worked together with a team to determines the architecture of a second subcomplex of the nuclear pore complex, in so doing solving a major quandary of answering how a nuclear pore complex (NPC) can be such an effective gatekeeper, preventing much from entering the nucleus while helping to shuttle certain molecules across the nuclear envelope.
The scientific team was featured in multiple publications including Caltech and the Argonne National Laboratory Science Highlights (reproduced in part below). The research was supported in part by the Consortium, and culminated in the following publication:
|
Architecture of the fungal nuclear pore inner ring complex Stuwe T, Bley CJ, Thierbach K, Petrovic S, Schilbach S, Mayo DJ, Perriches T, Rundlet EJ, Jeon YE, Collins LN, Huber FM, Lin DH, Paduch M, Koide A, Lu V, Fischer J, Hurt E, Koide S, Kossiakoff AA, Hoelz A. Science. 2015 Oct 2;350(6256):56-64. PMID: 26316600. |
Solving a Major Piece of a Cellular Mystery
This article by Argonne reproduced in part can be read here
 Not just anything is allowed to enter the nucleus, the heart of eukaryotic cells where, among other things, genetic information is stored. A double membrane, called the “nuclear envelope,” serves as a wall, protecting the contents of the nucleus. Any molecules trying to enter or exit the nucleus must do so via a cellular gatekeeper known as the nuclear pore complex (NPC), or pore, which exists within the envelope.
Not just anything is allowed to enter the nucleus, the heart of eukaryotic cells where, among other things, genetic information is stored. A double membrane, called the “nuclear envelope,” serves as a wall, protecting the contents of the nucleus. Any molecules trying to enter or exit the nucleus must do so via a cellular gatekeeper known as the nuclear pore complex (NPC), or pore, which exists within the envelope.
How can the NPC be such an effective gatekeeper — preventing much from entering the nucleus while helping to shuttle certain molecules across the nuclear envelope? Scientists have been trying to figure that out for decades, at least in part because the NPC is targeted by a number of diseases, including some aggressive forms of leukemia and nervous system disorders such as a hereditary form of Lou Gehrig’s disease. Now a team of researchers from Caltech, The University of Chicago, and the Biochemistry Center of Heidelberg University (Germany), led by André Hoelz and working at three U.S. Department of Energy synchrotron light sources including the Advanced Photon Source (APS) at Argonne, has solved a crucial piece of the puzzle.
In February of this 2015, the team published a paper describing the atomic structure of the NPC’s coat nucleoporin complex, a subcomplex that forms what they now call the outer rings (see the figure). Building on that work, the team has now solved the architecture of the pore’s inner ring, a subcomplex that is central to the NPC’s ability to serve as a barrier and transport facilitator. In order to determine that architecture, which determines how the ring’s proteins interact with each other, the biochemists built up the complex in a test tube and then systematically dissected it to understand the individual interactions between components. Then they validated that this is actually how it works in vivo, in a species of fungus.

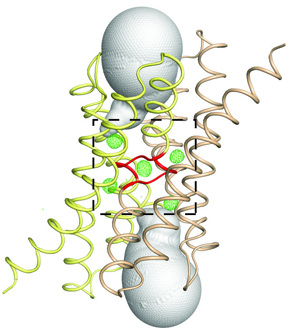 Fluoride protects our teeth against cavity-causing bacteria by making our teeth stronger. But what if we could find a way to trap fluoride ions (the negatively charged form of the chemical element fluorine) inside bacteria? At the right concentration, fluoride ions are highly toxic to bacteria, wreaking havoc on their proteins and disrupting critical cellular functions. Bacteria, however, can fight back, exporting the toxic fluoride ions out using specialized proteins called fluoride-specific ion channels.
Fluoride protects our teeth against cavity-causing bacteria by making our teeth stronger. But what if we could find a way to trap fluoride ions (the negatively charged form of the chemical element fluorine) inside bacteria? At the right concentration, fluoride ions are highly toxic to bacteria, wreaking havoc on their proteins and disrupting critical cellular functions. Bacteria, however, can fight back, exporting the toxic fluoride ions out using specialized proteins called fluoride-specific ion channels.





