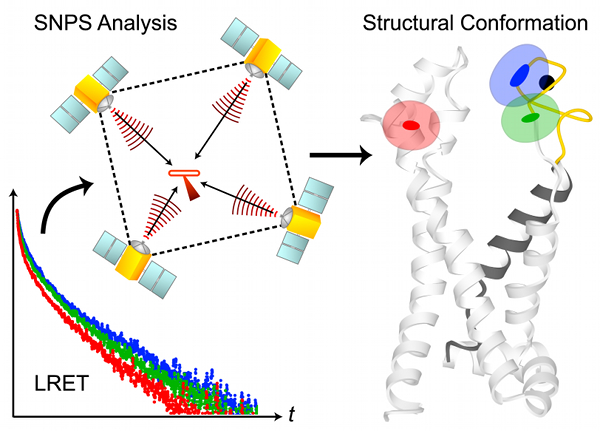
The Great Lakes Consortium for Petascale Computation has awarded access to the Blue Waters supercomputer — which is capable of performing quadrillions of calculations every second and of working with quadrillions of bytes of data — to 10 diverse science and engineering projects, including a project titled “The mechanism of the sarco/endoplasmic reticulum ATP-driven calcium pump”, spearheaded by Benoît Roux and his team.
The Great Lakes Consortium for Petascale Computation is a collaboration among colleges, universities, national research laboratories, and other educational institutions that facilitates the widespread and effective use of petascale computing. The computing and data capabilities of Blue Waters will assist researchers in addressing questions of biology, nanoelectronics, ecological and economic impacts of climate change, and more.
Roux’s work with the Blue Waters supercomputer will make a significant contribution to the Conformational Transitions in P-class ATPases Project of the Membrane Protein Structural Dynamics Consortium (MPSDC), in which Roux collaborates with Francisco Bezanilla. Roux’s team provided the following description of their research plans with Blue Waters:
Understanding the detailed molecular mechanism of ion pumps has been a long standing problem. In the early parts of the previous decade, a major breakthrough came in the form of determination of atomic resolution X-ray crystal structures of calcium transporting pump of sarcoplasmic reticulum of skeletal muscles (SERCA) that uses ATP hydrolysis as a source of free energy. Detailed structural studies of the pump under different conditions provided analogues of various intermediates in the reaction cycle and revealed important changes in the tertiary structure of the protein both in the cytoplasmic and in the transmembrane parts. Two major outstanding issues are the pathways of the ions to and from the transmembrane binding sites and a detailed understanding of the large scale conformational changes among various functionally relevant states. We will apply all-atom molecular dynamics (MD) and string method with swarms-of-trajectories to study transition pathways among various experimental structures.
The allocations provided on the Blue Waters supercomputer will allow us to study this important membrane protein with unprecedented detail. This study will reveal the molecular mechanism of an important step in the ion pumping process of a P-type ATPase and will provide a solid ground to understand other ATP-driven ion pumps such as the sodium-potassium pump, which shares very high sequence similarity with SERCA. ”
Congratulations to Benoît and his team for receiving this important award!