Membrane Protein Structural Dynamics Consortium (MPSDC) team member Hassane Mchaourab was recently featured in the Vanderbilt University Medical Center Reporter, along with a team of scientists who have linked a non-inherited, de novo mutation in the dopamine transporter to autism spectrum disorder (ASD).
The research was partially funded by the MPSDC, and contributes to the overall objectives of the Transport Cycle in Neurotransmitter Uptake Systems project in which Mchaourab is an active collaborator.
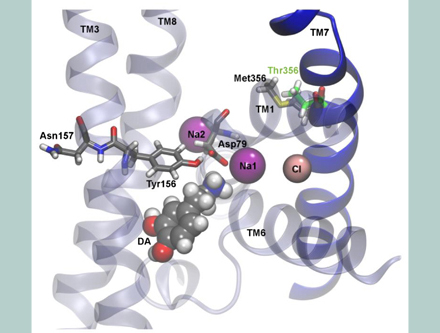
The group’s research was published in the journal Molecular Psychiatry, with Mchaourab as one of the senior authors. You can read more about the publication here.
Read the Vanderbilt University Medical Center Reporter after the jump.

Heiner Matthies, Ph.D., at the white board, leads a “seminar” for colleagues who hold vials of their fruit fly model that for the first time linked a non-inherited mutation in the dopamine transporter to autism. Seated at right, from the back, are Nicholas Campbell, Aurelio Galli, Ph.D., and P.J. Hamilton. Seated at left are Hassane Mchaourab, Ph.D., and James Sutcliffe, Ph.D. (photo by Susan Urmy)
Transporter linked to autism risk
Vanderbilt University investigators for the first time have linked a non-inherited, de novo mutation in the dopamine transporter to autism spectrum disorder (ASD).
The finding, published online Aug. 27 in the journal Molecular Psychiatry, suggests that altered dopamine signaling caused by a spontaneously occurring dopamine transporter malfunction may be a risk factor for ASD.
The de novo mutation was among several associated with ASD that were reported by a multi-center group of investigators, including three from Vanderbilt, last year in the journal Nature.
In the current paper, first authors P.J. Hamilton and Nicholas Campbell and their colleagues report that this particular mutation causes the dopamine transporter to basically work in reverse. Hamilton and Campbell are Ph.D. candidates in the Vanderbilt Brain Institute.
Normally the transporter regulates the supply of the neurotransmitter dopamine, which is involved in brain functions as diverse as motor activity, motivation, attention and reward, by removing excess dopamine from the synapse, or gap, between nerve cells.
The researchers showed that this mutation results an “efflux” or release of dopamine by the transporter into the synapse.
They found that fruit flies with the mutated transporter were hyperactive, a common behavioral characteristic of children with attention-deficit hyperactivity disorder (ADHD), which frequently co-occurs with ASD. This is the first animal model linking dysregulated dopamine transporter function associated with a de novo mutation and neuropsychiatric disorders.
The researchers concluded that abnormal dopamine release by its transporter may not only impact risk for ASD, but “may be an unappreciated source of risk for … (other) disorders associated with altered (dopamine) signaling,” including bipolar disorder, schizophrenia and ADHD.
The research team included scientists from the University of Copenhagen, Weill Cornell Medical College and the University of Illinois at Chicago.
Leading senior authors were Hassane Mchaourab, Ph.D., Heiner Matthies, Ph.D., James Sutcliffe, Ph.D., and Aurelio Galli, Ph.D., faculty members in the Vanderbilt Department of Molecular Physiology & Biophysics and the Neuroscience Program in Substance Abuse (N-PISA).
The research was supported by a National Science Foundation Graduate Research Fellowship and by National Institutes of Health grants DA035535, GM087519, HD055751, DA013975, MH089482 and DA012408.







