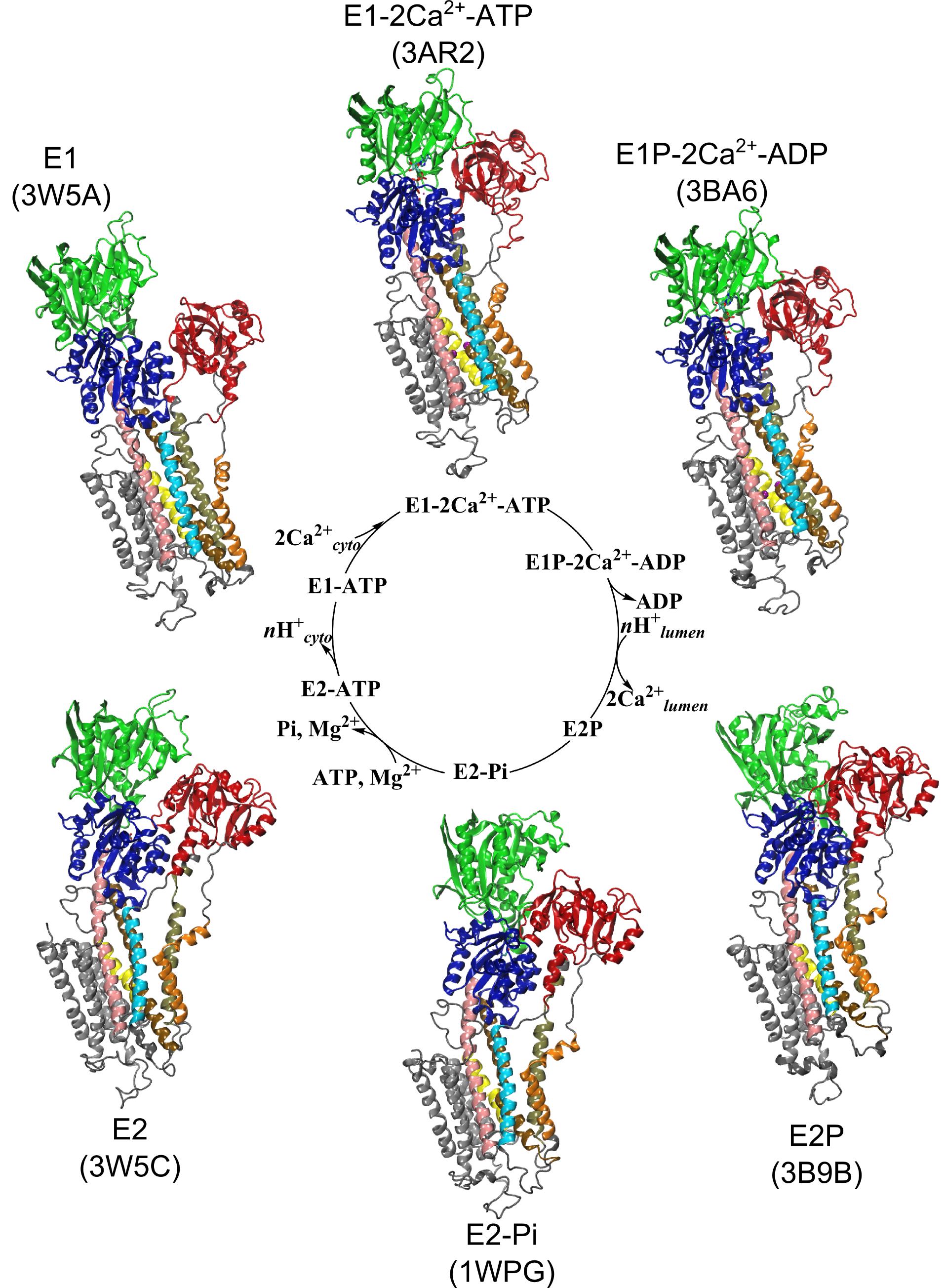
Friends,
As we get everything in order to welcome you this Friday to our Frontiers in Membrane Proteins Structural Dynamics Conference, we wanted to share with you the final program of the meeting.
The next few days promise to be a truly exceptional survey of membrane proteins structure and dynamics today. A veritable state of the art of the field. With our two keynote speakers (Chris Miller and Olga Boudker) leading 8 individual mini-symposia.
Personally, we are very much looking forward to seeing you and interacting with you later this week.
All the best,
The Organizing Committee
III Frontiers in membrane Protein Structural Dynamics
APS Conference Center, Argonne National Lab
Friday, November 10
| 7:45 | Continental Breakfast, APS 402 Atrium |
| 8:15 | Eduardo Perozo (University of Chicago), Stephen Streiffer (Advanced Photon Source) Welcome and overall perspective |
Session I: ABC Transporters and Partners: Dynamics at the Edge of Stability
| 8:25 | Christine Ziegler (Universität Regensburg) Introduction |
| 8:30 | Doug Rees (Caltech University) ABC transporters and the alt-access movement |
| 8:55 | Show-Ling Shyng (Oregon Health & Science University) Illumination of the SUR1-Kir6.2 partnership in KATP channels by single-particle cryo-EM |
| 9:20 | Heather Pinkett (Northwestern University) The role of ABC transporters in nutrient uptake and pathogenesis |
| 9:45 | Break, APS 402 Atrium |
Session II: Technology Advances in Protein Expression and Engineering
| 10:25 | Chris Ahern (University of Iowa) Introduction |
| 10:30 | Anthony Kossiakoff (University of Chicago) Synthetic Antibodies to facilitate structural analyses of functional conformational intermediates by both crystallography and Cryo-EM |
| 10:55 | Bill Clemons (Caltech University) Solving the membrane protein expression problem |
| 11:20 | Andrew Kruse (Harvard University) New technologies to interrogate membrane protein function |
| 11:45 | Lunch, APS 402 – Lower Gallery |
Session III: Pumping Ions and Other Flotsam
| 1:00 | Francisco Bezanilla (University of Chicago) Introduction |
| 1:05 | Bernd Fakler (University of Freiburg, Germany) Native PMCAs – proteomic surprises with old Ca2+-pumps |
| 1:30 | Miguel Holmgren (National Institutes of Health) Dynamics of the Na/K ATPase with the binding/unbinding of external Na ions |
| 1:55 | Huan Rui (University of Chicago) Probing the cycling mechanism of the P-type ATPase: Insights from kinetic modeling simulations |
| 2:20 | Break, APS 402 Atrium |
Session IV: Poster Rapid Fire
| 2:50 | Yeong-Kyun Shin (Iowa State University) Introduction |
| 2:55 | Poster presenters 2 minute talks |
Session V: Keynote Speaker 1
| 4:45 | A Brief Vignette Honoring Jean Chin (Eduardo Perozo, University of Chicago) |
| 5:00 | Alessio Accardi (Weill Cornell University) Introduction |
| 5:10 | Christopher Miller (Brandeis University) How channel crystallography made me stupid |
| 6:00 | Dinner, APS 402 – Lower Gallery Posters, APS 402 – E1100/E1200 |
Saturday, November 11
Session VI: Calculating Conformational Changes: At the Bleeding Edge
| 7:45 | Continental Breakfast, APS 402 Atrium |
| 8:25 | Wonpil Im (LeHigh University) Introduction |
| 8:30 | Benoit Roux (University of Chicago) C-type Inactivation and the Constricted-Like Conformations of the Selectivity Filter of K+ Channels |
| 8:55 | Michael Grabe (University of California, San Francisco) TMEM16 lipid scrambles bend membranes to get things done |
| 9:20 | Jose Faraldo-Gomez (National Institutes of Health) The Dos and Don’ts of the Alternating-Access Mechanism: Lessons from the Sodium-Calcium Exchanger |
| 9:45 | Break, APS 402 Atrium |
Session VII: Protein Motion Within a Field: Ins and Outs of Voltage-dependent Gating
| 10:25 | Sudha Chakrapani (Case Western Reserve University) Introduction |
| 10:30 | Jian Yang (Columbia University) Cryo-EM structure of a eukaryotic cyclic nucleotide-gated channel |
| 10:55 | Bonnie Wallace (Birbeck, University of London) Structural, Function and Disease-Related Aspects of Sodium Channel Voltage-Gating |
| 11:20 | Francisco Bezanilla (University of Chicago) Voltage sensors and membrane capacitance |
| 11:45 | Rama Ranganthan (University of Chicago) Protein mechanics: the link between structure, function, and evolution |
| 12:10 | Lunch, APS 402 – Lower Gallery |
Session VIII: Structure and Dynamics with Few Molecules (or a Lot)
| 1:25 | Valeria Vasquez (University of Tennessee, Memphis) Introduction |
| 1:30 | Robert Fischetti (APS, Argonne National Lab) Serial crystallography with monochromatic and polychromatic X-ray beams, and the APS Upgrade |
| 1:55 | Yeon-Kyun Shin (Iowa State University) Zooming in on single vehicle fusion |
| 2:20 | Simon Scheuring (Weill Cornell University) High-Speed Atomic Force Microscopy: A New Tool for the Study of the Dynamics of Single Unlabeled Membrane Proteins |
| 2:45 | Break, APS 402 Atrium Posters, APS 401 – E1100/E1200 |
Session IX: The Good, the Bad and the Ugly: Conformational Changes and the Transport/Translocation Cycle
| 4:30 | Ming Zhou (Baylor University) Introduction |
| 4:35 | Filipo Mancia (Columbia University) The Molecular Mechanisms Underlying Cellular Uptake of Vitamin A |
| 5:00 | Aurelio Galli (University of Vanderbilt) Failure to Prime the Dopamine ‘Pump’ in Autism |
| 5:25 | Alessio Accardi (Weill Cornell University) Exploring the backdoor of CLC channels and transporters: how a glutamate gets in and out of the Cl- permeation pathway |
| 5:50 | Dinner, APS 402 – Lower Gallery Posters, APS 401 – E1100/E1200 |
Sunday, November 12
Session X: Structural Dynamics Through Imaging
| 9:00 | Tobin Sosnick (University of Chicago) Introduction |
| 9:05 | Christine Ziegler (Universität Regensburg) Being in the right place: Localization-dependent lipid interactions in the PC2 TRP Channel |
| 9:30 | Irina Serysheva (University of Texas Houston) Structure of IP3R Channel: Towards Understanding Gating Mechanism |
| 9:55 | Vera Moiseenkova-Bell (University of Pennsylvania) Molecular mechanism of the TRPV2 channel pore dynamics during ligand activation |
| 10:20 | Mingley Zhao (University of Chicago) Molecular Mechanism of SNARE Complex Disassembly |
| 10:55 | Break, APS 402 – Atrium |
Session XI: Keynote Speaker 2
| 11:05 | Robert Nakamoto (University of Virginia) Introduction |
| 11:15 | Olga Boudker (Weill Cornell University) Dynamic underpinnings of transport mechanism in glutamate transporters |
Closing
*Shuttle service from the guest house to the conference center will start 15 minutes prior to the start of the meeting.
*Shuttle service from the conference center to the guest house will run for 15 minutes after the poster session (9:00pm).